Nonsurgical Penile Girth Enhancement with Soft Tissue Fillers -A Review of the Most Common Products
- 1. Avanti Derma, Tijuana, Mexico
Abstract
The quest for a larger penis has seemingly always been an obsession of the human male, and attempts to enlarge male genitalia date back to the dawn of human history and span cultures throughout the world.
CITATION
Casavantes LC, Morales P (2021) Nonsurgical Penile Girth Enhancement with Soft Tissue Fillers -A Review of the Most Common Products. JSM Sexual Med 5(1): 1065.
INTRODUCTION
The first incidences of effective surgical intervention was free fat transfer (FFT), more of a new application for autologous fat transfer, an existing surgical technique consisting of taking fat from one part of the body and moving it to another [1]. In theory, penile girth enhancement with FFT was a relatively straightforward procedure. Still, it soon became apparent that there were drawbacks that often had devastating consequences for men who had undergone the process. Further surgical attempts consisted of dermal fat grafts [2], allografts [3], and soft silicone implants [4], with advantages and sometimes severe drawbacks.
Surgeons have not reached a consensus to support anyone’s method. The surgical approach to penis enlargement remains a widely controversial subject, with only a tiny fraction of physicians prepared or willing to perform these surgeries.
Ultimately, the penis is a uniquely complex organ to augment due to its dynamic nature; its variations in size and consistency between its flaccid and erect states present the biggest challenge to find an appropriate implant material or method. The implant must be soft when flaccid yet firm when erect; it should also be able to stretch su?ciently to accommodate erections, and it must not interfere with the process of urination and ejaculation.
So the medical community was left with one conclusion to consider: Perhaps surgery is not the answer?
The arrival of soft tissue fillers presented a genuine alternative for penile girth enhancement: a nonsurgical option that delivered the desired results with a lower risk of complications.
Modern procedures have been moving away from aggressive surgical operations to in-o?ce methods that required no hospitalization, general anesthesia, incisions, drains, stitches, scars, or prolonged downtime. Necrosis, scarring, and deformity, which were common after surgical procedures, have been reduced to rare occurrences using correct injection techniques and soft tissue fillers.
The list of commercially available soft tissue fillers is diverse, and new products or variants are presented continuously. However, only a few of them are suitable for nonsurgical phalloplasty, and they offer essential differences that are important to understand.
The injector must have good knowledge and experience to choose the best option for each individual patient.
Soft tissue filler-based phalloplasty started in 2007 when surgeons from Brazil and Mexico [5] offered non-absorbable fillers (PMMA), but new products have made their way into this field.
The most popular soft tissue fillers for nonsurgical phalloplasty
In the author’s experience, there are only four soft tissue fillers that are safe and e?cacious for nonsurgical phalloplasty: calcium hydroxylapatite, hyaluronic acid, polycaprolactone, and polymethylmethacrylate, mentioned in alphabetical order.
The four products offer different options for physical characteristics, mechanism of action, longevity, firmness, and reversibility, so it is essential to understand the characteristics of each one. All of them are injected into the areolar space of the penis, between the superficial (Dartos) and the deep (Buck’s) fascias, using different injection techniques with micro- cannulas, needles, and injection guns.
None of these fillers require of skin sensitivity test or reconstitution. All of them are used off- label for nonsurgical phalloplasty.
Calcium hydroxylapatite (CaHA, Radiesse®)
The soft tissue filler comprises microspheres (diameter 25- 45 micrometers) of synthetic bone (calcium) suspended in a carboxymethylcellulose gel matrix [6]. The ratio CaHA / carrier are around 30% / 70%.
This product is widely used as a filler to correct moderate to severe wrinkles and as a natural collagen stimulator for skin rejuvenation and hydration [7].
There is no scientific documentation that supports the longevity of CaHA in phalloplasty patients, but the reports of facial procedures generally establish duration greater than one year.
The CaHA penile implant is firm to the touch. However, it tends to granulate (multiple modulations are palpable) when a high volume (> 12cc) of product is used or when more than one session is performed.
CaHA (commercialized as Radiesse®, Merz Pharma, Frankfurt, Germany) ranks fourth in popularity for nonsurgical phalloplasty.
Hyaluronic acid (HA, Juvederm®, Perfectha®, Belotero®, etc)
HA is a hydrophilic gel widely used as filler to correct wrinkles and small tissue deficits and as a volumizer for facial rejuvenation.
HA is the standard soft tissue filler for facial procedures used by most aesthetic injectors because it is a naturally occurring substance in our bodies and doesn’t trigger foreign bodies or late inflammatory reactions. HA has the added benefit that its effects are reversible with injections of hyaluronidase [8].
Nevertheless, penile HA implants are soft in consistency and feel spongy to palpation. Collagen formation is negligible, and HA remains a soft gel in the tissues; making the implant soft, spongy, and unstable; shifting and migration are pretty common due to the dynamics of the penis and the lassitude of the skin when the organ is at rest.
HA is manufactured and commercialized under multiple brands (Juvederm Voluma and Juvederm Volux by Allergan, Perfectha and Belotero by Merz Pharma, etc.). It ranks third in popularity for nonsurgical phalloplasty.
Polycaprolactone (PCL, Ellansé®)
This soft tissue filler is made of microspheres (diameter 25- 50 micrometers) of synthetic and latex-free polycaprolactone suspended in a carboxymethylcellulose gel matrix [9]. The ratio PCL / carrier are 30% / 70%.
PCL is the newest of the four products and has no US FDA approval yet. However, its popularity has spread rapidly in the European Union and other Latin American countries [de Melo].
In our practice, we observe that the duration of the PCL in phalloplasty patients goes well beyond the ranges reported by the manufacturer in face patients.
The PCL penile implant is naturally firm to palpation, and the results can be very satisfactory for most patients.
PCL (commercialized as Ellansé®, Merz Pharma, Frankfurt, Germany) ranks second in popularity. Still, it is growing rapidly, and it can be predicted that it will soon take the top spot as the most sought-after option for phalloplasty, especially after its US FDA approval.
Polymethylmethacrylate (PMMA)
PMMA is the oldest of the four products and has no US FDA approval for the formulation used for this report. This soft tissue filler is made of microspheres (diameter 40 micrometers) of synthetic polymethylmethacrylate suspended in a carboxymethylcellulose gel matrix [10]. It is available in three different concentrations with ratio PMMA / carrier 30%/ 70%, 10% / 90% and 2% / 98%. This soft tissue filler is popular in Latin America and some European countries.
PMMA is the pioneer product in nonsurgical phalloplasty and has been used for this purpose since 2007 [5].
The PMMA penile implant is naturally firm to palpation, and the results can be very satisfactory for most patients. The di?culty with PMMA is that being permanent soft tissue filler, blemishes and defects will also be permanent and occasionally require surgery to correct.
PMMA is commercialized as Linnea Safe® in Brazil and other countries and as Linnea Avanti® in Mexico (Lebon Laboratories, Porto Alegre, Brazil) ranks first in popularity. Still, it seems that PCL will put it in second place in the near future.
MECHANISM OF ACTION
Microsphere-based soft tissue fillers
Three of the soft tissue fillers mentioned in this review (CaHA, PCL, and PMMA) are collagen stimulants. They are composed of microspheres suspended in carboxymethylcellulose that is absorbed over time. The microspheres remain in place, and fibroblasts appear, triggering neocollagenesis [11].
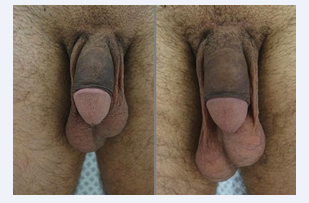
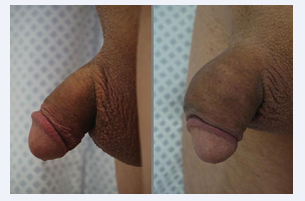
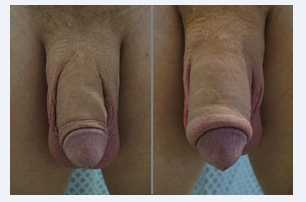
Eventually, each microsphere will be surrounded by new collagen fibers that ultimately create the bulking. Neo- vascularization also develops, which is why some authors [12] have called it a “live implant” because it behaves very similarly to normal skin or tissues (Figures 1-4).
Figure 1: Phalloplasty with Calcium Hydroxylapatite.
Figure 2: Phalloplasty with Hyaluronic Acid.
Figure 3: Phalloplasty with Polycaprolactone.
Figure 4: Phalloplasty with Polymethylmethacrylate.
Microsphere-based filler phalloplasty is very natural to the eye and to the touch.
Gel-based soft tissue fillers
The fourth product (HA) is considered a volumizer, and it has an entirely different mechanism of action. It is a hydrophilic gel that creates volume by retaining water and creating a viscoelastic solution. Neocolagenesis is minimal, and the implant remains in the tissues like a soft gel.
HA is a widely accepted soft tissue filler for facial wrinkles and small tissue deficits, and the popularity of high viscosity products has grown as an off-label indication for phalloplasty.
Probably the best attribute of HA is that it can be degraded immediately with the use of hyaluronidase, which is reassuring for those patients who may be unhappy with their results.
On the other hand, the main complaint from patients who have undergone phalloplasty with hyaluronic acid is that the implant feels abnormally soft and spongy.
The line between a dose su?cient to produce a noticeable increase in volume and an excessive amount that creates a spongy implant is very narrow. Still, we know that the spongy sensation increases with the quantity of filler used.
Longevity
The longevity of this group of products is dramatically different. PMMA is permanent as the microspheres are not phagocytosed or degraded and will remain in tissues for life; the other two microsphere-based products are considered long- lasting, and HA are temporary.
The effects of CaHA go beyond one year, and PCL is distributed in three alternatives: Ellansé-S one year, Ellansé-M two years, and Ellansé-L three years. A four-year version (Ellanse-E) has been discontinued or hasn’t been available in some countries outside the European Union.
HA, even in its most dense versions, has the shortest life span of the four products. In the author’s experience, a high percentage of treated patients report the disappearance of the implant in less than six months.
Firmness
The texture of the implant is one of the factors that can make a big difference in the level of patient satisfaction.
The HA implant is soft and spongy; the most common complaints in patients who have received this product are the spongy feeling and the short duration of the implant. The only recourse to avoid this is to use a low volume of the product, but this compromises the final girth increase.
PCL and PMMA produce a natural to rigid consistency implant. The use of moderate amounts of these products, especially if they are injected by an experienced physician, makes an implant that can go unnoticed to the touch. The use of large amounts or many sessions of these materials will create an unsightly implant that is noticeable to the touch and to the eye.
Reversibility
Of the four products, HA is the only one whose effects can be reversed immediately using hyaluronidase directly injected into the center of the implant [13].
PCL and CaHa disappear over time as they are metabolized by the body, and although they are not reversible, their effects and defects will gradually wear off.
PMMA is permanent and irreversible; the surgical approach is the only resource to correct imperfections caused by this product.
CONCLUSION
Like many other fields of aesthetic medicine, phalloplasty has undergone a rapid evolution, especially after the integration of injectable fillers that offer an outpatient procedure that avoids the use of the operating room, surgical cuts, sutures, drains, and scars with a significant reduction of morbidity and downtime.
Although to date we do not have perfect soft tissue filler, we have a range of products that can offer essential alternatives, from non-absorbable, permanent and non-reversible, to short- term and reversible absorbable. It will be fascinating to witness what lies ahead for nonsurgical phalloplasty; At the moment, this low-aggression in-o?ce procedure is becoming increasingly popular [14], and the results have proven to be very satisfactory (87%) [5].
REFERENCES
- Littara A, Melone R, Morales-Medina J, Tommaso Iannitti, Beniamino Palmieri. Cosmetic penile enhancement surgery: a 3- year single- centre retrospective clinical evaluation of 355 cases. Sci Rep. 2019; 9: 6323.
- Lisi Xu, Muxin Zhao, Wen Chen, Yangqun Li, Zhe Yang, Ning Ma, et al. Augmentation Phalloplasty With Autologous Dermal Fat Graft in the Treatment of “Small Penis”. Ann Plas Surg. 2016; 77: S60-5.
- Solomon M, Komlo C, Defrain M. Allograft materials in phalloplasty: a comparative analysis. Ann Plast Surg. 2013; 71: 297-9.
- Shirvanian V, Lemperle G, Araujo-Pinto C, Elist J. Elist implant review abstract. Int J Impot Res. 2014; 26: 100-4.
- Casavantes L, Lemperle G, Morales P. Penile Girth Enhancement with PMMA-Based Soft Tissue Fillers. J Sex Med. 2016; 13: 1414-22.
- Ridenour B, Kontis T. Injectable calcium hydroxylapatite microspheres (Radiesse). Facial Plast Surg. 2009; 25: 100-5.
- Lawrence S Bass, Stacy Smith, Mariano Busso, Marla McClaren. Calcium hydroxylapatite (Radiesse) for treatment of nasolabial folds: long-term safety and e?cacy results. Aesthet Surg J. 2010; 30: 235-8.
- Matarasso S. Understanding and using hyaluronic acid. Test Surg J. 2004; 24: 361-4.
- de Melo F, Nicolau P, Piovano L, Shang-Li Lin, Tiago Baptista- Fernandes, Martyn I King, et al. Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone-based collagen stimulator (Ellansé®). Clin Cosm Invest Derm. 2017; 10: 431-40.
- Gottfried Lemperle, Terry R Knapp, Neil S Sadick, Stefan M Lemperle. ArteFill permanent injectable for soft tissue augmentation: I. Mechanism of action and injection techniques. Aest Plast Surg. 2010; 34: 264-72.
- Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008; 34: S64-S67.
- Nacul A. Bioplastia. A plastica interativa. Ed Quintessence, 1st Edn. 2018.
- Cohen B, Bashey S, Wysong A. The use of hyaluronidase in Cosmetic Dermatology: A review of the literature. J Clin Investigat Dermatol. 2015; 3: 1-7.
- Oates J, Sharp G. Nonsurgical Medical Penile Girth Augmentation: Experience-Based Recommendations. Test Surg J. 2017; 37: 1032-38.