Possible Pre- and Postnatal Correction of Sexual Disorders in Individuals Prenatally Exposed to Maternal Immune Stress: An Interplay with GnRH Neuron Migration during the Prenatal Development
- 1. Koltsov Institute of Developmental Biology, Russian Academy of Sciences, Russia
Abstract
According to WHO (world health organization), approximatively 10% of couples of childbearing age suffer from infertility. The general causes and possible methods of restoring the reproductive possibility of both sexes are disputable. One of the factors that disrupt the development of the reproductive system is prenatal immune stress induced by bacterial infection during pregnancy.
Bacterial infection affecting the mother’s body during pregnancy can lead to various disorders in the development of the fetus without interrupting the course of pregnancy. The consequences can develop in children during further postnatal ontogenesis. The special attention could be paid to the possibility of correction of bacterial infection effect on the mother during the early stages of pregnancy to maintain reproductive health of offspring. The results of our studies, in combination with the existing literature data, determine two critical points of pre-and postnatal ontogenesis: 1) after exposure to bacterial infection at the end of first trimester of pregnancy; 2) in the early infantile period in children born in a result of first trimester bacterial infection complicated pregnancy.
We assume that risk of the infertility could be reduced if human development is controlled from the very beginning stage and treated of its complications not only by gynecologists but also by pediatricians who observe children after complicated pregnancy until his childbearing age. This review transfers the data obtained in animals (mainly rats and mice) in the field of early correction of human reproductive problems. This will give a more complete picture of the studies conducted on animals and extrapolate them to pre - and postnatal human ontogenesis.
Keywords
Pregnancy; Gonadotropin releasing hormone; Prenatal stress; Developmental disorders; Pro-inflammatory cytokines; Prenatal correction
Citation
Viktoria S, Marina I (2022) Possible Pre- and Postnatal Correction of Sexual Disorders in Individuals Prenatally Exposed to Maternal Immune Stress: An Interplay with GnRH Neuron Migration during the Prenatal Development. JSM Sexual Med 6(3): 1088.
INTRODUCTION
According to experimental and clinical data of recent decades, the effects of adverse environmental factors during critical periods of development of fetal physiological systems have a negative impact on their formation and functioning in adults [1-3]. Regulation of the formation of neuroendocrine, immune and reproductive systems is carried out with their close interaction already in early ontogenesis [4,5]. The effects of infectious factors on the mother’s body, pharmacological effects, improper nutrition and lifestyle of the mother during pregnancy can disrupt the functions of systems for a long time in postnatal life of offspring. The effects of various stressful stimuli on the mother can change the physiological concentrations of various mediators of the immune and neuroendocrine systems in fetuses and disrupt the regulatory mechanisms of development [5,6]. A significant risk factor that causes miscarriage or changes the structure and functions of the interacting systems of the fetal body is inflammation induced by infectious factors in the mother [7,8]. One of the targets for the negative effects of inflammatory products is the brain of a developing fetus [3,9]. Disorders of brain development lead to further maladaptation, and in some cases – disability of children. After birth, their risk of developing diseases such as schizophrenia, depression, and autism increases [3,10-12]. Changes occurring in the brain can also lead to disorders of the development of the hypothalamicpituitary-gonadal axis (HPG), a decrease in reproductive ability or infertility in sexually mature offspring [12-15]. In childbearing age, treatment of gonadal functions impaired in early development is often ineffective. In this regard, for the timely prevention of stress-induced disorders in people in the pre- and early postnatal periods, it is necessary to conduct research on experimental models that allow determining critical periods of development, specific signaling molecules and mechanisms of their action. The processes of formation of physiological systems are characterized by sensitivity to many regulatory factors. This article presents an analysis of our own and literature data on the negative impact of various stress stimuli on the development and functioning of the neuroendocrine, immune and reproductive systems and on approaches to prenatal correction of disorders in offspring caused by inflammatory processes in early ontogenesis.
GnRH as a Main Hypothalamic Peptide Hormone of Reproductive Axis Regulation in Vertebrates
Decapeptide GnRH is crucial in the regulation of reproductive function in adult mammals. The various vertebrate species have revealed a variety of forms of GnRH, which are expressed not only in neurons, but also in various peripheral tissues and have different origins, structure and functional significance [16]. Outside the brain, GnRH is found in lymphoid tissue, liver, heart, skeletal muscles, kidneys, mammary gland, placenta, testes, ovaries, prostate of various animal and human species [17,18].
The assumptions about the presence of various forms of GnRH were expressed as early as the mid-80s of the last century and were based on obtaining separate fractions of GnRH. The large number of genes encoding GnRH and the corresponding peptide sequences in different species revealed in the future required the introduction of a general, universal and informative nomenclature. In 1997, the Genome Database Nomenclature Comity established the following names: the hypothalamic form of GnRH-I, the mesencephalic form of GnRH-II and the telencephalic form of GNRH-III found in fish (Fernald and White, 1999). Under the name GnRH neurons (GnRH system), as a rule, also is mined a population of hypothalamus neurons expressing GnRH I.
The GnRH system of most mammals is a diffuse cluster of neurons located in the septo-preoptic region and the anterior hypothalamus. Despite some species differences, the distribution of GnRH-containing neurons in the vertebrate brain follows two basic principles: (1) neurons do not form their own isolated clusters and are not located within known anatomical nuclei; (2) most of them lie near the midline from the olfactory bulbs to the ventral hypothalamus. The total number of GnRH-containing neurons is about 1200-1400. Most of the axons of these neurons end in the median eminence, where GnRH, being released into the portal blood circulation system, regulates the synthesis and secretion by gonadotropocytes of luteinizing (LH) and follicle-stimulating (FSH) hormones, which, in turn, regulate the secretion of sex hormones. The axons of the GnRH neurons secrete GnRH into the portal system with a certain frequency, creating in it the concentration necessary to start the secretion of LH and FSH. Despite the abundance of data on the effect of individual neurotransmitters on the release of GnRH, there is no clear hypothesis explaining this mechanism. According to some authors, the mechanism of pulsative release of GNRH is embedded in the neurons themselves [19], while according to others, this mechanism may include a network of different neurons that act on GnRH neurons through separate or multiple neuronal systems. This hypothesis also assumes a certain periodicity in the functioning of the GnRH neurons themselves [20]. Along with the modulation of gonadotropins, GnRH also modulates sexual behavior, olfactory signal transmission, formation and functioning of the immune system [16,21].
The GnRH system of adult mammals, in turn, is under complex neuroendocrine control. A huge neural network, including neurotransmitters (gamma-aminobutyric acid - GABA, norepinephrine, dopamine, and serotonin), opioid peptides, endorphins, kisspeptin, leptin, etc., mediates the influence of external stimuli on the state of the reproductive system through its main link – GnRH neurons. Special attention in this complex regulation is focused on the interactions of the GnRH and immune systems. Stimulation of the immune system induced by bacterial infection causes suppression of the synthesis and release of GNRH [22,23]. The body thus reduces unnecessary functions and focuses on the fight against foreign infections and its survival.
GnRH Neurons Origin and Migration in Mammals
In vertebrates, most of the GnRH neurons are formed in the prenatal period outside the brain from the epithelium of olfactory placodes [24]. Next, the GnRH neurons migrate to the forebrain, where they are located in adults. The migration of GnRH neurons in the nasal part of the head occurs along the terminal, vomeronasal and olfactory nerves, after penetrating into the brain through the cribriform plate of the ethmoid bone, GnRH neurons continue to migrate to the septal region of the forebrain along the trajectory of the terminal nerve [25]. In most mammalian species, the overall picture of the development of the GnRH system remains similar, although there are regular differences in the time of formation and migration of neurons, determined by the timing of pregnancy and the degree of maturity of the fetus at the time of birth in different species [24,26]. The processes of migration of GnRH neurons are usually divided into three stages: (1) intranasal migration, (2) penetration through the cribriform plate of the ethmoid bone, and (3) intracerebral migration. On each of them, a unique set of cell adhesion proteins, gradients of guiding molecules, microenvironment of cells producing neurotransmitters and neuromodulators necessary for successful migration are formed in normal development. Complete disruption of the migration of GnRH neurons, leads to underdevelopment of the gonads (hypogonadotropic hypogonadism hypogonadotropic hypogonadism) and lack of sense of smell in humans (anosmia anosmia), known as Kallman syndrome [27]. Slowing down the migration of GnRH neurons can lead to the fact that GNRH neurons, getting into the forebrain later, will not be able to form the necessary axonal connections and take their place in the reproductive axis in mature individuals.
It should be noted that a large number of studies demonstrating the influence of various factors on the migration of GnRH neurons were conducted on models in which knockout mice (knock-out mutations) were used. Thus, a violation of the migration of GnRH neurons was detected in fetuses of mice with a knockout of the gene controlling the expression of the key enzyme of GABA synthesis (GAD67) [28]. Pharmacological shutdown or stimulation of the synthesis of regulatory molecules affecting the development of the GnRH system in fetuses is also often used as an experimental model [29,30], or the introduction of their agonists or antagonists [31].
However, only a small number of studies is aimed at studying disorders in the formation of the entire hypothalamic-gonadal axis and the formation of reproductive function.
Effects of Different Signal Molecules on Normal Development of GnRH Neurons
In the last 20 years, a huge layer of disparate data has been accumulated on the influence of various factors on the development of GnRH neurons. These signaling molecules are usually divided into groups according to their functional significance: (1) transcription factors, (2) cell adhesion molecules, (3) soluble guiding factors, and (4) neurohormones.
Taking into account the origin of GnRH neurons in olfactory placodes, it was assumed that morphogenetic factors involved in the differentiation of olfactory placodes could control the isolation of a population of progenitor cells of GnRH neurons from the mass of cells forming the olfactory epithelium. The initial stages of olfactory placode differentiation are controlled by several transcription factors: OTX-1 and -2 [32], Pax-6 [33], Eya-1 and -2 [34], six-3 [35]. The OTX-2 homeoprotein (a product of the otx2 homeobox gene), involved in the development of head structures in vertebrates at early stages of development, was detected immunocytochemically in mouse GnRH neurons [36]. The authors suggest that this protein is involved in the process of initiation and directed migration of cells. Other transcription factors can also, apparently, directly control the formation of a population of GnRH neurons, however, their expression in these neurons has not yet been shown by direct methods. At the same time, various transcription factors are expressed in different types of epithelium. Thus, the development of the olfactory epithelium is controlled by the expression of a cascade of transcription factors Mash-1, Math4A, Math4/neurogenin1, NeuroD [37]. Later, the expression of transcription factors involved in brain development - Olf-1 and GATA-4 - was also described in olfactory epithelial cells [38,39]. These data suggest that the precursors of GnRH neurons are formed from a group of cells at the junction between the olfactory and respiratory epithelium [40].
The migration of GnRH neurons in the nasal region of the head is closely related to the olfactory, terminal and vomeronasal nerves. After penetration into the forebrain of the GnRH, neurons follow the trajectory of one of the temporal projections of the vomeronasal nerve, directed to the septo-preoptic region [41]. In rats, the migration of GnRH neurons in the nasal region of the head is closely related to bundles of nerve fibers expressing polysialized forms of nerve cell adhesion proteins (PSA-NCAM) [41]. Various experiments on the removal of NCAM from the migration pathway of GnRH neurons (knockout of genes controlling synthesis, enzymatic removal or administration of antibodies to NCAM) have shown that such disorders, despite a significant decrease in the number of migrating GnRH neurons, do not lead to a complete blockade of their migration [42]. In mice, the migration of GnRH neurons is associated with bundles of nerve fibers expressing the protein of intermediate filaments – peripherin [43].
In the development of the olfactory system, the participation of a large number of so–called guide molecules - chemoattractants, chemotrophic chemorepellents and factors such as slit proteins [44], semaphorins [45], netrins [46], have been described. These proteins direct the growth of axons of olfactory nerves and some of them are supposed to be involved in the direction of migration of GnRH neurons in the nasal region of the head and in the forebrain. Moreover, peripherin that expressed along nerve fibers in mouse is essential for the migration of GnRH neurons in the nasal region of the head. In addition, another chemoattractant protein, hepatocyte growth factor HGF/SF, has shown to participate in the direction of the migration of GNRH neurons in the nasal region [47]. Recently, the involvement of stromal chemokine cell factor 1 (SDF-1) and one of its receptors, CXCR4, in the migration of GnRH neurons has been shown [48]. It is been assumed that SDF-1 accelerates the intranasal migration of GnRH neurons by changing the sodium level in migrating neurons [49].
It is also been noted that cytokines are involved in the development of olfactory structures and migration of GNRH neurons: leukemia inhibiting factor (leukemia inhibiting factor - LIF) and monocyte chemotaxis protein (beta-chemokine, monocyte chemoattractant protein-1 - MCP-1). Expression of LIF and its LIF receptor was been detected in the nasal region of 13-day-old fetuses of mice. It was shown that LIF induces their chemokinesis on the model of immature and migration-capable GN11 neurons [50]. Expression of MCP-1 and its receptor, the CC-2 motif receptor (CCR2) was been detected in GnRH neurons of the hypothalamus, as well as in GT1-7 and Gn11 cell lines. In the culture of these cells, MCP-1 stimulates the migration of immature GNRH neurons [51]. The level of cytokines increases significantly, when the fetus is exposed to bacterial endotoxin LPS, which leads to disorders in the development of the brain, including the GNRH system.
Neurotransmitters mainly control the penetration of GnRH neurons into the forebrain. In mice, a decrease in the rate of migration of GnRH neurons occurs in the area of the lattice plate of the lattice bone against the background of an increase in the concentration of intracellular calcium as a result of tonic depolarization of neurons induced by GABA [52,53]. It is possible that GnRH neurons use this period for their maturation or migration behavior during entering the forebrain. GABA also affects the spread of GnRH neurons in the forebrain. Another neurotransmitter affecting the migration of GnRH neurons is glutamate. The introduction of an AMPA receptor blocker to pregnant female mice leads to a delay in the penetration of GnRH neurons into the forebrain in fetuses [54]. Monoamines are also modulators of the migration of GnRH neurons. Serotonin deficiency models in rat fetuses have shown that serotonin stimulates the proliferation and further migration of GnRH neurons to the forebrain [29]. Suppression of catecholamine synthesis in rat embryos by alpha-methyl-p-tyrosine (αMPT, a competitive TH inhibitor), starting from the 11th day of development, leads to increased numbers of GnRH neurons in the rostral segments of their migration pathway by the 17th day and their accumulation in the zone of their penetration into the forebrain on the 18th-21st day [30].
Development of the GnRH System in Different Pathological States: with Special Attention to Maternal Immune Stress
Regulation of the formation of various functions in a developing organism is been carried out by a single complex supersystem, including neuroendocrine, immune and reproductive systems [21]. Programming the interactions of these systems with various stressful stimuli during critical periods of their development leads to the emergence of various neuropsychiatric, metabolic, immunological and reproductive disorders in offspring [3,6,9,55]. Women often have asymptomatic diseases caused by bacteria, which is especially dangerous during the gestation period. Recent data indicate an increase in the number of women unable to independently carry and give birth to a healthy child. The main target for the negative effects of inflammatory products in the mother during critical periods of ontogenesis is the brain of her developing fetus.
Over the past 5 years, the number of studies on the involvement of the immune system in the development of neurodegenerative disease - Parkinson’s disease has been steadily increasing. It is assumed that some form of early symptoms of PD are hereditary, while a later manifestation of the disease is associated with the negative impact of environmental factors, including neuroinflammation, on the development of dopamine-mediated error system of the fetus [56].
Along with brain development disorders, activation of the mother’s immune system by bacteria, most often gram-negative, in the early stages of pregnancy can lead to disorders of the formation of the reproductive system in both men and women and the development of infertility in adulthood [13,15,57]. In this regard, the treatment of couples suffering from infertility associated with insufficient gonad function is often not effective.
The effect of prenatal inflammation on the development of pathologies of the reproductive system in offspring whose mothers were exposed to immune activation at different stages of pregnancy has been studied less intensively. As is known, the functioning of the hypothalamic-pituitary-gonadal (HPG) axis of an adult organism is determined by the interaction of neurons of the anterior hypothalamus, secreting GnRH, pituitary gonadotropocytes, secreting gonadotropic hormones (LH and FSH) and steroidogenic gonadal cells (Leydig cells in the testes and cells of the inner lining, granulosa cells of the follicle in the ovaries).
The first studies on the effect of inflammation induced by high doses of LPS (250 mcg/kg) administered to rats on the 21st day of pregnancy revealed a link between activation of the mother’s immune system and disorders in the development of sexual behavior in male offspring [58]. Subsequently, it was shown that the administration of LPS to female mice in low doses (1-10 mcg / kg) on the 10th day of pregnancy leads to a decrease in the level of LH and testosterone in the blood, and as a consequence, to the suppression of reproductive ability in male offspring [59]. Prolonged administration of LPS from the 13 th to the 17th days of pregnancy leads to impaired testicular development and a decrease in testosterone levels in the blood of adult offspring of mice [12]. According to our data, the administration of LPS (45 mcg / kg) to female mice on the 12th day of pregnancy, during the initial period of intranasal migration of GnRH neurons to the forebrain, leads to a slowdown, but not a cessation, of their migration rates. IL-6 receptors have been identified on the path of intranasal migration of GNRH neurons on nerves sprouting into the brain [57]. LPS provoked maternal inflammation could have misbalancing effect on the highly organized early steps of GnRH neuronal migration in nasal compartment that leads to decreasing of GnRH-producing neuron number at later stages of development.
Activation of the mother’s immune system of LPS leads to an increase in the synthesis of pro- and anti-inflammatory cytokines. Their level increases in the placenta, amniotic fluid and on the periphery during the first 2 hours after injection [57,60]. In the brain and peripheral blood of fetuses, there is also a significant increase in the content of proinflammatory cytokines - TNFα, IL-1β, IL-6, monocyte chemotactic protein MCP-1, leukemia inhibitory factor (LIF), which have a negative effect on the developing organism, including the fetal HPG system [57,61]. Disorders of the pituitary gland and gonads can lead to the development of reproductive potential disorders in the postnatal offspring of such mothers.
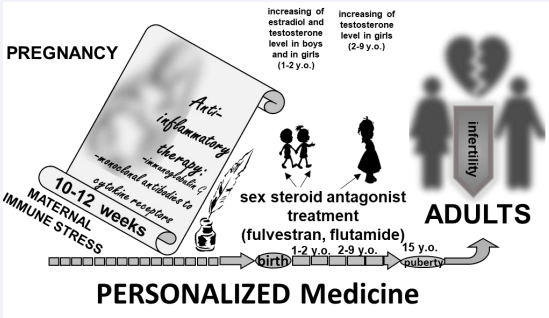
Later, we obtained data in favor of the fact that violations of the development of testes and ovaries in female and male rats after the immune activation of the mother of LPS on the 12th day of pregnancy are associated largely with an increase in the level of steroid hormones during early postnatal ontogenesis. Thus, when a testosterone receptor antagonist (flutamide) is administered from the 14th to the 30th days of postnatal development to females or an estradiol receptor antagonist (fulvestran) from the 5th to the 14th days to males, almost complete restoration of reproductive ability in offspring has been occurred [14,15]. Disorders of testicular and ovarian development against the background of elevated levels of steroid hormones without LPS administration has been studied previously. We assumed that LPS introduction in rats in the early stages of pregnancy leads to the suppression of the synthesis of GnRH and LH, which determine the reproductive cycles in sexually mature offspring, but also to more significant violations of the formation of normal development of the testes and ovaries, which, in turn, could been corrected in the postnatal period.
Approaches to the Prevention or Cancellation of Disorders Induced by Acute Inflammation in Early Ontogenesis in Mammals
The development of inflammatory processes induced by activation of the immune system in different periods of ontogenesis can have a negative impact on both the formation and functioning of various brain systems. Bacterial infection affecting the mother’s body during pregnancy can lead to various disorders in the development of the fetus without interrupting the course of pregnancy, and the consequences of these disorders can develop in children throughout postnatal ontogenesis.
To preserve reproductive health in men and women of childbearing age, we focus on the possibility of correcting the effects of bacterial infection of the mother in the early stages of pregnancy (the first trimester in humans).
Thus, we define two critical periods of control and correction of the development of reproductive health in humans: the end of the first trimester of pregnancy (Figure 1) and the period of early infantilism in children born because of such a complicated pregnancy
Figure 1: Possible options for correcting defects in the development of the human reproductive axis caused by bacterial infection of the mother at the end of the first trimester of pregnancy.
Because of our work, a clear system of support for people who are potentially disadvantaged in the long-term development of reproductive health and a system for organizing timely correction of the development of their reproductive abilities are determined. The presented data will be able to correct the actions of doctors of various specializations (gynecologists, neonatologists, pediatricians, and reproductologists) and integrate the direction of world medical practice aimed at preserving the reproductive health of the population. We would also like to identify some medicinal substances that could correct the reproductive health risks of the forming person in the process of his pre- and postnatal ontogenesis.
This concept defines the development and implementation of social programs aimed at educating the population about reproductive self-preservation behavior, as well as, most importantly, the development and implementation of special means for correcting the effects of bacterial infection of the mother during pregnancy (immunoglobulins, antibodies to pro-inflammatory cytokines synthesized in the mother’s body in response to immune stress). Children born because of such a pregnancy also need postnatal screening of the content of sex hormones, and possible correction of their production and content in the blood, with the help of methodological approaches possible in pediatrics, necessary for the successful formation of their reproductive health in the future.
The work was conducted under the IDB RAS Government basic research program in 2021 No 0088-2021-0008
REFERENCES
4. Zakharova LA, Izvolskaia MS. Interactions between the reproductive and immune systems during ontogenesis: the role of GnRH, sex steroids and immunomediators. In: Kahn S.M., ed. Sex steroids. Zagreb: InTech; 2012: 341-370.
7. Chistyakova GN, Gazieva IA, Remizova II, Cherdantseva GA. Evaluation of cytokine production in pregnancy complicated by the threat of miscarriage in the first trimester. Fundamental Res. 2005; 5: 96-98.
21. Zakharova LA, Izvolskaia MS. In book: Sex Steroids Interactions between the reproductive and immune systems during ontogenesis: the role of GnRH, sex steroids and immunomediators. 2012: 341-70. InTech, Zagreb.
41. Yoshida K, Tobet SA, Crandall JE, Jimenez TP, Schwarting GA. The migration of luteinising hormone-releasing hormone neurons in the developing rat is associated with a transient, caudal projection of the vomeronasal nerve. J Neurosci. 1995; 15: 7769-7777.