Research Progress on the Neural Circuitry of Erectile Dysfunction
- 1. TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, China
- 2. Department of Neurology, Hospital of Chengdu University of Traditional Chinese Medicine, China
- 3. The Second Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, China
- 4. The Third Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, China
Abstract
Erectile dysfunction [ED] is a prevalent clinical condition significantly impacting the well-being and quality of life of males. Prior research has detected anomalies in the structural and functional networks of various brain regions, particularly within the limbic system, including the amygdala, in ED patients. As neuroscience progresses, the pivotal role of neural circuits in the pathogenesis of ED is becoming increasingly clear. Specifically, Kisspeptin neurons in the amygdala can regulate the neuroendocrine of HPG axis by activating GnRH neurons in the hypothalamus, thus affecting erectile function. Recent studies have also highlighted the BNST-POA neural circuit as a key regulator of male sexual behavior and the initiation of sexual function. This review synthesizes the latest insights into brain regions and neural circuits associated with ED, aiming to provide foundational knowledge for understanding the central mechanisms driving ED pathogenesis and to identify potential novel therapeutic targets.
Keywords
• Erectile dysfunction; Neuroimaging; Neural circuits; Central mechanisms; Research progress
CITATION
Yu M, Liu M, Zhang T, Zeng H, Li Z, et al. (2024) Research Progress on the Neural Circuitry of Erectile Dysfunction. JSM Sexual Med 8(3): 1137
INTRODUCTION
Penile erection is a dynamic physiological and psychological response process that relies on extensive brain functional areas, integrating cognition, emotion, and physiological activities [1]. The incidence of erectile dysfunction [ED] is high and has been increasing annually, with a positive correlation with age [2,3]. Currently, more than 100 million people worldwide suffer from ED, and studies predict that by 2025, over 300 million people will be affected by this condition [4]. Although ED does not directly threaten patients’ lives, it is closely related to age, cardiovascular diseases, depression, anxiety, diabetes, etc. These factors interact with each other, severely affecting men’s health and family quality of life, and further impacting social stability [5-7]. It is currently a significant disease affecting men’s health.
Sexual response involves multiple systems of the human body and is an extremely complex process [8,9]. Previous studies using brain magnetic resonance imaging to explore the mechanisms of sexual arousal have identified numerous supraspinal control centers related to penile erection, including the prefrontal cortex, parietal cortex, cingulate cortex, insula, caudate nucleus, putamen, thalamus, amygdala, and hypothalamus [10]. However, the specific mechanisms have yet to be further elucidated. Recently, important neural circuits involved in sexual physiological activities have been discovered, which control sex recognition, sexual arousal, and the subsequent mating behaviors and pleasure.
STRUCTURAL AND FUNCTIONAL BRAIN STUDIES RELATED TO ED
Microstructural Abnormalities in Brain Regions of ED Patients
The regulation of erectile function is complex, involving extensive central networks related to behavior, emotion, and cognition.Several studies have found characteristic changes in the structural networks of multiple brain regions in ED patients, mainly including changes in cortical thickness and structural abnormalities in white matter connections.Zhao et al. found that patients with psychogenic erectile dysfunction [pED] had widespread cortical thinning in multiple brain regions, including the medial prefrontal cortex, orbitofrontal cortex, cingulate gyrus, inferior temporal gyrus, and insular cortex. This reduction in cortical thickness was significantly associated with decreased male sexual function [11]. Zhang et al. found microstructural changes in the corpus callosum [genu, body, and splenium], corticospinal tract, internal capsule, corona radiata, external capsule, and superior longitudinal fasciculus white matter tracts in pED patients [12]. Another study examined the changes in the integrity, number, and length of white matter fibers and their myelin sheaths in the topological space connected to different brain regions in psychogenic ED. It found that the integrity of white matter fibers connecting the left prefrontal cortex and amygdala was impaired in pED patients. The degree centrality value of the left amygdala was negatively correlated with erectile function, suggesting that decreased information transmission in the left amygdala may be a potential factor in the pathogenesis of psychogenic ED [13].
Studies Based on Brain Functional Networks: The occurrence of ED is not only related to decreased connectivity between different brain regions but also closely associated with functional activity in these regions. Overactivation of certain brain areas can also negatively impact erection.Studies have shown that after visual erotic stimulation, patients with psychogenic ED exhibit greater activation in the left superior parietal lobe compared to healthy controls, especially in the later stages of sexual response, suggesting an important role of the left superior parietal lobe in the inhibition of sexual activity [14]. In healthy middle-aged men, visual erotic stimulation mainly activates the occipital-temporal region, anterior cingulate gyrus, insula, orbitofrontal cortex, and caudate nucleus [15]. Other studies have shown that in the default-mode network [DMN] subsystem, patients with psychogenic ED have decreased connectivity in the inferior parietal lobule, posterior cingulate cortex, and medial prefrontal cortex [16]. This subsystem is related to self- referential mental simulations, which involve recalling past experiences, contemplating the future, and imagining others’ perspectives. Studies by Xiang et al. based on the amplitude of low-frequency fluctuations revealed that patients with diabetes mellitus-related ED [DMED] had decreased fractional amplitude of low-frequency fluctuations [fALFF] in the left superior frontal gyrus [medial], middle temporal gyrus, and middle temporal pole, while the right postcentral gyrus showed increased fALFF [17]. DM-ED patients showed increased regional homogeneity [ReHo] in the right middle frontal gyrus, while decreased ReHo in the left superior frontal gyrus [dorsolateral], paracentral lobule, precuneus, and bilateral supplementary motor area. Moreover, ReHo values in the left superior frontal gyrus [dorsolateral] were significantly negatively correlated with IIEF-5 scores, indicating characteristic changes in the central functional networks of DMED patients [18].
Limbic System-Dominant Brain Regions as Key Central Nervous Areas for ED: The limbic system is a high-level integrative center for sexual behavior, regulating sexual arousal and desire. Emotional stimuli, psychological states, and cognitive function networks play important regulatory roles in erectile function. ED patients exhibit functional connectivity abnormalities primarily in limbic system-related brain regions. Studies have reported that in the limbic system of pED patients, the fALFF is reduced in the left medial superior frontal gyrus, left lingual gyrus, left putamen, and right putamen, with functional changes in the medial superior frontal gyrus and caudate nucleus, which are correlated with sexual function and psychological status. Multiple studies have demonstrated that erectile function is closely related to emotional and cognitive regulatory networks. The low-frequency fluctuations [ALFF] in the right anterior insula of pED patients are related to changes in erectile function, suggesting impaired cognitive and motivational processing of sexual stimuli in pED patients [19]. Psychogenic ED patients exhibit reduced functional activity in the left dorsolateral prefrontal cortex and decreased functional connectivity between nuclei in the limbic system, which is significantly associated with the sexual function and psychosocial status of pED patients [20,21]. The amygdala is one of the more primitive nuclei in the brain and a key nucleus in the limbic system, associated with basic instinctive activities. Sexual activity is one of these instinctive activities, gradually formed and consolidated during biological evolution, primarily determined by genetics, such as copulatory ability and fertility [22]. Additionally, the amygdala is responsible for fear and emotional recognition, and its activation helps biological individuals monitor environmental stimuli [23]. Damage to the connectivity function of the amygdala may impair emotional regulation, increase individual alertness, inhibit sexual arousal and pleasure during sexual activity, and thus lead to ED [Table 1] [24-34] presents an overview of the research on the regulatory mechanisms of the central network involved in erectile dysfunction.]
Table.1 Presents research that utilizes neuroimaging techniques to investigate the central mechanisms of erectile dysfunction. The studies have identified structural and functional abnormalities in brain regions associated with erectile function, including the prefrontal cortex, parietal cortex, cingulate cortex, insula, caudate nucleus, putamen, thalamus, amygdala, and hypothalamus.
|
Related research on the regulation mechanism of erectile dysfunction central network |
|||||
|
ID |
Date |
Author |
Technology |
Brain regions and their characteristic changes |
Functions |
|
1 |
2003 |
Hagemann, JörnH.etc[24] |
PET |
Increased brain activity in the anterior cingulate gyrus and right prefrontal cortex and decreased activity in the temporal cortex |
Activate penis erection |
|
2 |
2006 |
Kim, S W.etc[15] |
fMRI |
The main activation areas were occipital temporal region, anterior cingulate gyrus, insula, orbitofrontal cortex and caudate nucleus. |
Regulating the sexual arousal of visual stimuli |
|
3 |
2006 |
Yang Bo.etc[25] |
fMRI |
Compared with normal men, the activation range of bilateral anterior cingulate gyrus in patients with psychological ED is larger. |
Regulating the sexual arousal of visual stimuli |
|
4 |
2008 |
Wang Tao.etc[26] |
PET |
The metabolic changes of 18F-fluorodeoxyglucose (18F-FDG) in bilateral hypothalamus of ED patients were not obvious. |
Regulating the inhibition of sexual response |
|
5 |
2012 |
Cera, Nicoletta.etc[14] |
fMRI |
Increased activation of the left superior parietal lobe |
|
|
6 |
2014 |
Cera, Nicoletta.etc[16] |
fMRI |
In the default-mode network (DMN) mode, the connectivity values of the inferior parietal lobe, posterior cingulate cortex and medial prefrontal cortex decreased. In the salience network (SN) model, decreased connectivity and increased connectivity were observed in the right insula and anterior cingulate cortex, respectively. |
self relevant mental simulation recognition of autonomical and sexual arousal changes |
|
7 |
2014 |
Zhang, Peihai.etc[12] |
DTI |
the psychological ED patients showed increased FA values, reduced MD values and reduced AD values in multiple WM tracts including the corpus callosum (genu, body and splenium), corticospinal tract, internal capsule, corona radiata, external capsule and superior longitudinal fasciculus |
|
|
8 |
2015 |
Liu, Qi.etc[27] |
fMRI |
Increased brain activity in bilateral cerebellum, insula, globus pallidus, parahippocampal gyrus, orbitofrontal cortex (OFC) and middle cingulate cortex (MCC). |
|
|
9 |
2017 |
Chen, J.etc[28] |
fMRI |
The pathlength and intensity of the right superior frontal gyrus (dorsolateral), superior parietal gyrus, parahippocampal gyrus and left temporal pole (superior temporal gyrus), post-central gyrus changed. |
Regulating cognitive and emotional processes |
|
10 |
2017 |
Chen Jianhuai.etc[13] |
DTI |
The integrity of the white matter fiber myelin sheath connected to the left frontal lobe and amygdala in patients with psychological ED is impaired. |
|
|
11 |
2018 |
Chen, Guotao.etc[29] |
fMRI |
Characteristic changes were found in amygdala, dorsal thalamus, hypothalamus, caudate putamen, cingulate gyrus, insular cortex, visual cortex, sensory cortex, motor cortex and cerebellum. |
|
|
12 |
2018 |
Jin, Chenwang.etc[19] |
fMRI |
Baseline brain activity was significantly lower in the right anterior insula and right orbital frontal cortex of pED patients. |
|
|
13 |
2018 |
Li, Lingli.etc[30] |
DTI |
The cortical volume of the left postcentral gyrus and precentral gyrus was significantly reducedin ED patients, while only the cortical volume of the right middle temporal gyrus was significantly increased. |
|
|
14 |
2020 |
Yin, Tao.etc[20] |
fMRI |
The Amplitude of Low Frequency Fluctuations (ALFF) of the left dorsolateral prefrontal cortex (dlPFC) and the functional connectivity (FC) between the left dlPFC and the left angular gyrus, left posterior cingulate gyrus (PCC) and precuneus were decreasedin ED patients. |
|
|
15 |
2020 |
Yin Tao.etc[31] |
fMRI |
The ReHo value of the left lateral cerebellar region increased and the ReHo value of the right precentral gyrus decreased in PED patients. |
|
|
16 |
2022 |
Chen, Jianhuai.etc[18] |
fMRI |
In the DM-ED group, the ReHo value of the right middle frontal gyrus increased, and the ReHo values of the left superior frontal gyrus ( dorsolateral ), paracentral lobule, precuneus and bilateral supplementary motor area decreased. |
|
|
17 |
2023 |
Feng, Sitong.etc[32] |
DTI |
pED patients showed higher fractional anisotropy (FA) values between left transverse temporal sulcus and left supramarginal gyrus, and lower FA values between left suborbital sulcus and left para-hippocampal part of the medial occipito-temporal gyrus. |
|
|
18 |
2023 |
Liu, Xue.etc[33] |
fMRI |
There are brain function changes in the medial superior frontal gyrus and caudate putamen in pED patients. |
Regulating sexual function and psychological state |
|
19 |
2023 |
Yang, Yuqing.etc[34] |
fMRI |
PED patients showed increased fALFF in the right posterior cingulate cortex (PCC), right dorsolateral prefrontal cortex (DLPFC), right supplementary motor area (SMA) and left middle occipital gyrus. |
Regulating the inhibition of sexual response |
|
20 |
2024 |
Xiang, Ziliang.etc[17] |
fMRI |
The fractional amplitude of low frequency fluctuation in the left superior frontal gyrus (medial), middle temporal gyrus and middle temporal gyrus (pole)decreased, and the ALFF index in the right postcentral gyrus increased. |
|
The role of neural circuits in erectile dysfunction diseases
The basal nucleus neurons of the amygdala project excitatory fibers to the shell of the nucleus accumbens, forming an essential neural circuit for reward mechanisms [35]. The bed nucleus of the stria terminalis [BNST] within the extended amygdala is a crucial structure in the limbic system, associated with the generation of anxiety and the initiation of male sexual behavior [36-38]. Testosterone can also modulate the monoamine oxidase and catechol-O-methyltransferase in the amygdala and other limbic brain regions involved in depression and mediation of antidepressant responses [38]. The medial preoptic area of the hypothalamus can regulate male sexual behavior [39,40], and chemogenetic activation of the POA brain region can induce penile erection and increase the frequency of erections by fourfold compared to normal levels [40]. Olfactory information from the opposite sex is an important means of conveying social and sexual information between animals, and the amygdala’s projection of neurons to the hypothalamus completes olfactory- mediated mate selection activities, which is a key prerequisite for regulating reproductive behavior [41].
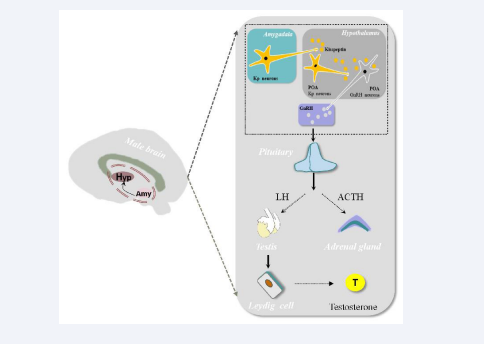
Activation of Amygdala Kisspeptin Neurons Regulates Hypothalamic Preoptic Area GnRH Neurons in the Hypothalamic-Pituitary-Gonadal Axis: The hypothalamus and amygdala play important roles in the regulation of emotions and behavior. In rodents, the accessory olfactory system has fiber projections to amygdala Kisspeptin neurons, and amygdala Kisspeptin neurons project to GnRH neurons in the hypothalamic preoptic area [POA] as well as to POA Kisspeptin neurons. This neural circuit reveals the neuroanatomical structure linking amygdala Kisspeptin neurons, olfactory pheromones, and the HPG axis [42]. In humans, Kisspeptin neuron groups in the hypothalamic arcuate nucleus [ARC] and POA send fiber projections to GnRH neurons at the basal hypothalamus [43]. After the Kisspeptin protein matures, it reaches the medial preoptic area containing many GnRH-synthesizing and secreting neurons, binds with receptor protein 54 [GPR54], which then binds with G-protein coupled receptors on these neurons, promoting Kisspeptin’s function to stimulate GnRH secretion [44]. GnRH synthesized and secreted by GnRH neurons enters the anterior pituitary via the hypothalamic-pituitary portal system. GnRH acts on gonadotropin-secreting cells, stimulating the secretion of LH and FSH, which then act on the male testes to promote the secretion of testosterone [T]. This, in turn, regulates the production of peripheral target steroid hormones, forming the HPG axis that controls reproductive organ development and gametogenesis [45-47] [shown in Figure 1].
Figure 1: The Kisspeptin neurons in the amygdala project to the gonadotropin-releasing hormone (GnRH) neurons in the preoptic area (POA) of the hypothalamus, as well as to the Kisspeptin neurons in the POA. The Kisspeptin protein secreted by these neurons can act on the GnRH neurons in the POA, stimulating them to secrete GnRH. GnRH acts on the gonadotropin-secreting cells, stimulating the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which in turn act on the male testes to stimulate the secretion of androgens, thereby initiating a series of biological effects.
Testosterone can reduce the secretion of GnRH and pituitary gonadotropins, and defects in the HPG axis can lead to gonadal dysfunction [48,49]. Testosterone induces the production of NO by activating eNOS [50]. Therefore, testosterone replacement therapy [TRT] can improve endothelial function and prevent ED, suggesting that endogenous testosterone can protect cavernous endothelium [51].
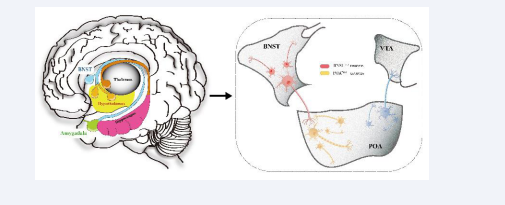
The Bed Nucleus of the Stria Terminalis–Hypothalamic Medial Preoptic Area [BNST-POA] is an Important Neural Circuit for Initiating Sexual Behavior and Function: The reward circuit for sexual behavior is a key mechanism discovered in recent years that influences sexual behavior. Subcortical brain areas, including the hypothalamus and caudate nucleus, are involved in sexual response, and the nucleus accumbens plays a role in pleasure and reward [52]. The bed nucleus of the stria terminalis estrogen receptor 1 [BNSTprTac1] is a basal forebrain structure located posterior to the nucleus accumbens, anterior to the thalamus, medial to the dorsal striatum, dorsal to the globus pallidus, and preoptic area. BNST is crucial for emotion and sexual behavior and is an important neural fiber connecting the nucleus accumbens, globus pallidus, and thalamus. The BNST- POA neural circuit controls gender recognition, sexual arousal, and the subsequent mating behavior and pleasure [53] as shown in Figure 2.
Figure 2: Shows the neuronal projection relationship of the BNST-POA neural circuit. After BNST recognizes the potential sexual partner through the received chemical sensing signal, the signal is input to the POATacr1 neuron (marked with orange) through the BNSTTac1 neuron (marked with red). The POA further inputs the signal to the ventral tegmental area (VTA ).
The BNST-POA is an important neural circuit for initiating sexual behavior and function. The BNST, part of the extended amygdala, is an important structure in the limbic system and is associated with the initiation of male sexual behavior.
BNST tachykinin precursor 1 neurons [BNSTTac1] can use chemosensory signals to distinguish gender, guiding male-female mating [36,38]. The hypothalamic medial preoptic area [POA] regulates male sexual behavior [41,54], and activation of the POA brain region through chemogenetic methods can induce penile erection and increase the number of erections fourfold compared to normal levels [38]. After BNST recognizes potential mates through chemosensory signals, it sends signals to the POA while releasing substance P to enhance excitatory transmission to POA neurons. The POA further sends signals to the periaqueductal gray [PAG] and ventral tegmental area [VTA], activating projections from the POA to the VTA. The nucleus accumbens [NAc] releases dopamine, triggering reward behavior. The POA is a prominent target of BNSTTac1 neurons. Optogenetic activation of hypothalamic medial preoptic area tachykinin receptor 1 neurons [POATacr1] significantly shortens the mating latency of male mice, producing timely mating behavior and increasing the number of mating events sixfold during the illumination period. Activating POATacr1 neurons post-ejaculation restores mating desire, reducing the refractory period from about five days to one second [38].
CONCLUSION
ED is a common male disorder that significantly impacts men’s psychology and life. Sexual physiological activities are directly regulated by the nervous system, and neural circuits are central mechanisms involved in the regulation of emotions, sexual behavior, and reproductive behavior. The amygdala and hypothalamus are important nuclei in the limbic system, capable of regulating depression and anxiety. Recent studies have discovered that Kisspeptin neurons in the amygdala and GnRH neurons in the hypothalamic preoptic area participate in regulating mating behavior. Amygdala Kisspeptin neurons and hypothalamic GnRH neurons are central neural nuclei for regulating sexual behavior and erectile function. Additionally, the BNST-POA is an important neural circuit for initiating sexual behavior and function. Therefore, studying ED from the perspective of neural circuits is of significant importance.
ACKNOWLEDGEMENT
In the Acknowledgement section, authors may include individuals, who are not listed as authors, and organizations that have made substantive contributions to the research or the manuscript. An exception is where funding was provided, which should be included in Funding Sources.
FUNDING SOURCES
This work was supported by Chengdu University of Traditional Chinese Medicine “Xinglin Scholars” Discipline Talent Scientific Research Improvement Program [Grant number MPRC2023016] and Study on Traditional Chinese Medicine Intervention Program for the Treatment of Delayed Hypogonadism by Tonifying Kidney and Replenishing Qi Method [grant number 2023MS454]. The fundings supported the selection of the research topic, its execution, as well as the conception, writing, and decision to publish the manuscript.
AUTHOR CONTRIBUTIONS
Meijun Liu and Maobin Yu summarized and revised the raw materials, and also created the tables and figures. Tao Zhang, Zilong Xu, Hongsen Zeng, and Ze Li helped retrieve and organize the literature. Peihai Zhang selected the research topic and designed the research program. Maobin Yu and Meijun Liu wrote the manuscript.
REFERENCES
- Graf H, Walter M, Metzger CD, Abler B. Antidepressant-related sexual dysfunction - perspectives from neuroimaging. Pharmacol Biochem Behav. 2014; 121: 138-145.
- Mola JR. Erectile Dysfunction in the Older Adult Male. Urol Nurs. 2015; 35: 87-93.
- Zhang MJ, Zhang CY, Jin BF, Shang XJ, Bin B, Wang YH, et al. Guidelines for diagnosis and treatment of premature ejaculation with integrated traditional Chinese and western medicine ( trial version ). National Journal of Andrology. 2018; 24: 176-181.
- Goldstein I, Burnett AL, Rosen RC, Park PW, Stecher VJ. The Serendipitous Story of Sildenafil: An Unexpected Oral Therapy for Erectile Dysfunction. Sex Med Rev. 2019; 7: 115-128.
- Sarma AV, Hotaling JM, de Boer IH, Dunn RL, Oerline MK, Singh K, et al. Blood pressure, antihypertensive medication use, and risk of erectile dysfunction in men with type I diabetes. J Hypertens. 2019; 37: 1070-1076.
- Manolis A, Doumas M, Ferri C, Mancia G. Erectile dysfunction and adherence to antihypertensive therapy: Focus on β-blockers. Eur J Intern Med. 2020; 81: 1-6.
- Nutalapati S, Ghagane SC, Nerli RB, Jali MV, Dixit NS. Association of erectile dysfunction and type II diabetes mellitus at a tertiary care centre of south India. Diabetes Metab Synd. 2020; 14: 649-653.
- Poeppl TB, Langguth B, Laird AR, Eickhoff SB. The functional neuroanatomy of male psychosexual and physiosexual arousal: a quantitative meta-analysis. Hum Brain Mapp. 2014; 35: 1404-1421.
- Janssen E. Sexual arousal in men: a review and conceptual analysis. Horm Behav. 2011; 59:708-716.
- Stoléru S, Fonteille V, Cornélis C, Joyal C, Moulier V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci Biobehav Rev. 2012; 36: 1481-1509.
- Zhao L, Guan M, Zhang X, Karama S, Khundrakpam B, Wang M, et al. Structural insights into aberrant cortical morphometry and network organization in psychogenic erectile dysfunction. Hum Brain Mapp. 2015; 36: 4469-4482.
- Zhang P, Liu J, Li G, Pan J, Li Z, Liu Q, et al. White matter microstructural changes in psychogenic erectile dysfunction patients. Andrology. 2014; 2: 379-385.
- Chen JH, Chen GT, Chen Y, Yao ZJ, Lu Q, Dai YT. [Correlation of abnormal topological properties of the white matter fibers connecting the left amygdale with psychogenic erectile dysfunction]. Zhonghua Nan Ke Xue. 2017; 23: 323-328.
- Cera N, Di Pierro ED, Sepede G, Gambi F, Perrucci MG, Merla A, et al. The role of left superior parietal lobe in male sexual behavior: dynamics of distinct components revealed by FMRI. J Sex Med. 2012; 9: 1602-1612.
- Kim SW, Sohn DW, Cho YH, Yang WS, Lee KU, Juh R, et al. Brain activation by visual erotic stimuli in healthy middle aged males. Int J Impot Res. 2006; 18: 452-457.
- Cera N, Di Pierro ED, Ferretti A, Tartaro A, Romani GL, Perrucci MG. Brain networks during free viewing of complex erotic movie: new insights on psychogenic erectile dysfunction. PLoS One. 2014; 9: e105336.
- Xiang Z, Huang Y, Xu Y, Liu X, Huang X, Liu T, et al. Altered brain activity in diabetic patients with erectile dysfunction revealed by fractional amplitude of low-frequency fluctuation: A resting-state fMRI study. Andrology. 2024; 12: 68-74.
- Chen J, Huang X, Tang Q, Xiang Z, Xu Y, Liu T, et al. Altered Regional Homogeneity in Patients With Diabetic Erectile Dysfunction: A Resting-State fMRI Study. Front Endocrinol (Lausanne). 2022; 13: 817523.
- Jin C, Guan M, Dong M, Wu J, He Z, Chen X, et al. Aberrant baseline brain activity in psychogenic erectile dysfunction patients: a resting state fMRI study. Brain Imaging Behav. 2018; 12: 1393-1404.
- Yin T, Liu Q, Ma Z, Li Z, Sun R, Ren F, et al. Associations Between Altered Cerebral Activity Patterns and Psychosocial Disorders in Patients With Psychogenic Erectile Dysfunction: A Mediation Analysis of fMRI. Front Psychiatry. 2020; 11: 583619.
- Ren FQ. The Study on the central Regulation Mechanism of Chaihu- Shugan Powder Influencing on the Therapeutic Effect of Treating for Liver-gi Stagnation-type Psychological Erectile Dysfunction [D] 2020.
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Ditzen B, Heiman JR. Lower sexual interest in postpartum women: relationship to amygdala activation and intranasal oxytocin. Horm Behav. 2013; 63: 114-121.
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014; 17: 106-113.
- Hagemann JH, Berding G, Bergh S, Sleep DJ, Knapp WH, Jonas U, et al. Effects of visual sexual stimuli and apomorphine SL on cerebral activity in men with erectile dysfunction. Eur Urol. 2003; 43: 412- 420.
- Yang B, Zhang JS, Zhou YC, Zhu WZ, Wang T, Xia LM, et al. fMRl study of psychogenic erectile dysfunction. Chinese Journal of Medical Imaging Technology. 2006; 11: 1638-41.
- Wang T, Liu B, Wu ZJ, Yang B, Liu JH, Wang JK, et al. Hypothalamus may be involved in psychogenic erectile dysfunction. Zhonghua Nan Ke Xue. 2008; 7: 602-605.
- Liu Q, Zhang P, Pan J, Li Z, Liu J, Li G, et al. Cerebral Activity Changes in Different Traditional Chinese Medicine Patterns of Psychogenic Erectile Dysfunction Patients. Evid-Based Compl Alternat. Med 2015; 2015: 503536.
- Chen J, Chen Y, Chen G, Dai Y, Yao Z, Lu Q. Altered brain networks in psychogenic erectile dysfunction: a resting-state fMRI study. Andrology. 2017; 5: 1073-1081.
- Chen G, Yang B, Chen J, Zhu L, Jiang H, Yu W, et al. Changes in Male Rat Sexual Behavior and Brain Activity Revealed by Functional Magnetic Resonance Imaging in Response to Chronic Mild Stress. J Sex Med. 2018; 15: 136-147.
- Li L, Fan W, Li J, Li Q, Wang J, Fan Y, et al. Abnormal brain structure as a potential biomarker for venous erectile dysfunction: evidence from multimodal MRI and machine learning. Eur Radiol. 2018; 28: 3789-3800.
- Yin T, Ren FQ, Ma ZY, Huang XP, Chang DG, Zhang PH. [Alterations of regional homogeneity in patients with psychogenic erectile dysfunction: A study by resting-state functional MRI]. Zhonghua Nan Ke Xue. 2020; 26: 118-122.
- Feng S, Dong L, Yan B, Zheng S, Feng Z, Li K, et al. Altered White Matter Structural Network Connectivity Associated with Cognitive Declines in Psychogenic Erectile Dysfunction. Neuroscience. 2023; 529: 54-61.
- Liu X, Liu S, Liu T, Tang L, Ji M, Xu Y, et al. Altered regional brain activity and functional connectivity in resting-state brain networks associated with psychological erectile dysfunction. Front Neurosci. 2023; 17: 1074327.
- Yang Y, Qu L, Mu L, Yao J, Su C, Zheng Q, et al. Electroacupuncture for psychogenic erectile dysfunction: A resting-state functional magnetic resonance imaging study exploring the alteration of fractional amplitude of low frequency fluctuation. Front Hum Neurosci. 2023; 17: 1116202.
- Vachez YM, Tooley JR, Abiraman K, Matikainen-Ankney B, Casey E, Earnest T, et al. Ventral arkypallidal neurons inhibit accumbal firing to promote reward consumption. Nat Neurosci. 2021; 24: 379-390.
- Haynes SE, Han MH. A Novel Role for Hypothalamic AgRP Neurons in Mediating Depressive Behavior. Trends neurosci. 2021; 44: 243-246.
- Knoedler JR, Inoue S, Bayless DW, Yang T, Tantry A, Davis CH, et al. A functional cellular framework for sex and estrous cycle-dependent gene expression and behavior. Cell. 2022; 185: 654-671.e22.
- Bayless DW, Yang T, Mason MM, Susanto AAT, Lobdell A, Shah NM. Limbic Neurons Shape Sex Recognition and Social Behavior in Sexually Naive Males. Cell. 2019; 176: 1190-1205.e20.
- Wei YC, Wang SR, Jiao ZL, Zhang W, Lin JK, Li XY, et al. Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat Commun. 2018; 9: 279.
- Kranz GS, Spies M, Vraka C, Kaufmann U, Klebermass EM, Handschuh PA, et al. High-dose testosterone treatment reduces monoamine oxidase A levels in the human brain: A preliminary report. Psychoneuroendocrino. 2021; 133: 105381.
- Guimaraes EL, Dias DO, Hau WF, Julien A, Holl D, Garcia-Collado M, et al. Corpora cavernosa fibroblasts mediate penile erection. Science. 2024; 383: eade8064.
- Pineda R, Plaisier F, Millar RP, Ludwig M. Amygdala Kisspeptin Neurons: Putative Mediators of Olfactory Control of the Gonadotropic Axis. Neuroendocrinology. 2017; 104: 223-238.
- Hrabovszky E, Molnár CS, Sipos MT, Vida B, Ciofi P, Borsay BA, et al. Sexual dimorphism of kisspeptin and neurokinin B immunoreactive neurons in the infundibular nucleus of aged men and women. Front Endocrinol (Lausanne). 2011; 2: 80.
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005; 25: 11349-11356.
- Celik O, Celik N, Aydin S, Aygun BK, Haberal ET, Kuloglu T, et al. Ghrelin action on GnRH neurons and pituitary gonadotropes might be mediated by GnIH-GPR147 system. Horm Mol Biol Clin Investig. 2016; 25: 121-128.
- Pinheiro VG, Cury JR, Satrapa RA, Trinca LA, Loureiro B, Barros CM. Evaluation of the hypothalamus-pituitary axis response to exogenous GnRH, estradiol benzoate, and LH during the postpartum period in Nellore cows. Theriogenology. 2013; 79: 797-802.
- Yang SH, Wei M, Su W, Cheng LY, Qiao YB, editors. Leptin regulates the hypothalamic-pituitary-gonadal axis via GnIH neurons. Annual Meeting of Chinese Anatomy Society; Xining, Qinghai, China. 2015- 08-08.
- Jin JM, Yang WX. Molecular regulation of hypothalamus-pituitary- gonads axis in males. Gene. 2014; 551: 15-25.
- Corradi PF, Corradi RB, Greene LW. Physiology of the Hypothalamic Pituitary Gonadal Axis in the Male. Urol Clin n Am. 2016; 43: 151-162.
- Yu J, Akishita M, Eto M, Ogawa S, Son BK, Kato S, et al. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology. 2010; 151: 1822-1828.
- Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urology. 2005; 174: 827-834.
- Ottenheimer DJ, Bari BA, Sutlief E, Fraser KM, Kim TH, Richard JM, et al. A quantitative reward prediction error signal in the ventral pallidum. Nat Neurosci. 2020; 23: 1267-1276.
- Bayless DW, Davis CO, Yang R, Wei Y, de Andrade Carvalho VM, Knoedler JR, et al. A neural circuit for male sexual behavior and reward. Cell. 2023; 186: 3862-3881.e28.
- Karigo T, Kennedy A, Yang B, Liu M, Tai D, Wahle IA, et al. Distinct hypothalamic control of same- and opposite-sex mounting behaviour in mice. Nature. 2021; 589: 258-263.