Severe Impairment of Reproductive Parameters and Hippocampus Structure after Chronic Stress Induction in Rats: The Impact of a Watch-Out Period
- 1. Department of Animal Organisms Biology, University of Douala, Cameroon
- 2. Department of Zoology and Entomology, University of Pretoria, South Africa
- 3. Department of Biology, University of Bamenda, Cameroon
- 4. Department of Biochemistry, University of Yaounde I, Cameroon
- 5. Department of Animal Biology, University of Dschang, Cameroon
Abstract
This study aimed at determining the effects of restrain and cold stress on neuroreproductive parameters in rats and assessing the effects of a watch out period. 36 adult rats were divided into 3 groups (n=12): not stressed, restraint and cold stressed (8 hours/day/4 weeks) during which, exploration, anxiety, sexual behaviour and body mass were evaluated. After the stress induction, the half of animals in each group were sacrificed while the second half was maintained during a watch-out period of 4 weeks before being sacrificed. After sacrifices, adrenal mass, biochemical, sperm and histomorphometric parameters of the testes and hippocampus were examined. Results showed that compared to the control group, restraint and cold stress caused significant decreases (p<0.05–0.001) in exploratory activities, body mass, sexual motivation and performance parameters, sperm count, mobility and daily sperm production, serum testosterone and total cholesterol levels coupled to significant increases (p<0.05 – 0.001) in anxiety parameters and adrenals gland mass. Significant reductions (p<0.05 – 0.001) in the number of cells in the Ammon’s horns 1 and 3 of the hippocampus and the diameter and epithelium height of the testicular seminiferous tubes were also observed. These alterations were more severe in restraint-stressed animals. After the watch-out period, the altered adrenal gland mass, exploration, anxiety and sexual behaviour parameters were restored whereas biochemical, sperm and histological parameters were not. These findings indicate that stress-induced neuroreproductive alterations depend on the nature of parameters, the characteristics of the stressor and could be used for the management of patients suffering from stress-induced infertility
Keywords
• Stress; Anxiety; Spermatogenesis; Testosterone; Ammon’s Horns; Rat
INTRODUCTION
More than 185 million people worldwide are affected by infertility and, according to the World Health Organization, 8-12% of couples worldwide are affected by this problem, with 50% of the causes attributed to a male factor [1,2]. Since the 1980s, a number of studies have shown that male fertility is falling sharply as a result of several factors [3]. In order to find solutions to this problem, these different causal factors need to be studied. Previous data suggests that male infertility is due to sperm deficiency, hormonal disorders, erectile/ejaculatory dysfunction or environmental factors such as psychological stress [4]. Stress is a non-specific response by the body to environmental factors that cause a disruption in homeostasis, resulting in physiological, behavioral and emotional changes that characterize the adaptations of the stress system [5,6]. These adaptations of the stress system depend on the type of stressor (physical, psychological), the duration or intensity of the stress and the body’s genetic predisposition to stress [7]. “Acute stress” is when a stressor is applied for a few hours, “subacute stress” when the stressor is repeated daily for several days and “chronic stress” if it lasts for weeks or months [5]. The Hypothalamic-Pituitary-Adrenal (HPA) axis and the Locus Ceruleus-Norepinephrine (LC/NE) system are the main components involved in the stress response [8]. The physiological response to stress begins with the central perception of a disturbance in homeostasis, which stimulates the Sympathetic Nervous System (SNS) and then the HPA axis, which produce epinephrine and glucocorticoid hormones respectively [8]. Major and/ or chronic stress factors, combined with inadequate responses to stress, can have adverse effects on a number of physiological functions, including growth, metabolism, the brain and reproduction [9-11]. Stress can affect various neuronal functions and structures in the brain, such as the hippocampus, amygdala and prefrontal cortex, since stress hormone receptors were later discovered in these areas [12,13]. Located in the medial temporal lobe of the brain, the hippocampus is essential for cognitive functions such as learning, memory and the regulation of behaviour [14,15]. Male infertility is a global public health problem for which research or studies to fully understand its extent and prevalence have not yet been fully carried out. Furthermore, although stress has long been recognized as a factor that can impair reproductive function, the exact pathophysiological and molecular mechanisms that explain this effect remain a major research challenge [10]. The search for the underlying aetiology and treatment of male infertility must therefore be thorough. Psychological stress, one of the most common and least understood causes of male infertility, has become a frequently cited factor in discussions on unexplained male reproductive failure [11,16,17]. Regarding the duration of stress on reproductive functions in males of many species, acute stress impairs reproduction if it occurs at a critical time in the precise time course of endocrine events that induce testosterone and/or sperm production [18]. During acute stress, the Hypothalamic-Pituitary-Adrenal (HPA) axis exerts a direct inhibitory effect on the Hypothalamic Pituitary-Gonadal (HPG) axis by inhibiting the synthesis of GnRH and its Receptor (GnRHR), disrupting the release of LH from the pituitary gland and increasing the function of Gonadotropin Inhibitory Hormone (GnIH) neurons [19]. However, chronic stress induces a generalised dysregulation of the stress response, leading to chronic hyperexcitation, with hyperactivation of the HPA axis and SNS and relative immunosuppression with marked hypersecretion of CRH, which may be involved in initiating and/or maintaining a pathophysiological vicious circle leading to impaired nervous and reproductive function/ structure [20,21], via various mechanisms that need to be studied and well elucidated.
Many stress-inducing experimental procedures have been identified and used in both humans and animals [22-24]. At present, studies demonstrating the precise neuroendocrine mechanisms involved in the development of stress-induced reproductive dysfunction are limited. In addition, most studies evaluating the effects of stress after a stress-induced procedure have failed to compare the effects of many types of stressors, particularly restraint and cold stress, or the effects of a watch-out period after the induction of chronic stress. Undoubtedly, a comparative study of different types of stressors, coupled with the observation of a watch-out period after stress induction, could provide an important basis for a better explanation of the physiological mechanisms by which chronic psychological stress impairs neuroreproductive function/structure in humans. Therefore, we hypothesised that four weeks of chronic stress could differentially affect the structure and/or disrupt many mechanisms of neuroreproductive function in rats and that a period of observation could probably restore the altered parameters, depending on the type of stressor, its intensity and the parameter altered. Therefore, the aim of the present study was to determine the effects of two types of stressors (restraint and cold) on some neurobiological and reproductive parameters in rats subjected to stress, and to study these parameters after cessation of the stressor. We performed behavioural observations, fertility tests (sperm parameters, testes and hippocampus histology, testosterone levels and oxidative stress assessments) in rats, starting with the assessment of anxiogenesis using the open field test and the light/dark box test, which are widely accepted as behavioural assays of anxiogenic behaviour in rodents
MATERIALS AND METHODS
Animals and ethical considerations
A total of 56 adult (3 months old) albino Wistar rats were used in this study, 36 males (210-230 g) for stress induction and 20 ovariectomised females (160-200 g) for evaluation of copulatory activity. These animals were housed in groups of six per round plastic cage (diameter: 39 cm, height: 13 cm) with wood shavings as bedding material in the animal housing unit of the Department of Biology of Animal Organisms, Faculty of Science, University of Douala (Cameroon). These animals were acclimatized in the experimental room for one week before the start of the experiments. These rats were maintained at room temperature with a natural light/dark cycle (12 h:12 h) and fed standard laboratory rat chow and water ad libitum. There were no human endpoints in this study.
All animal experiments carried out in this study were in accordance with the UK Animals (Scientific Procedures) Act, 1986 and the guidelines of the European Union Directive 2010/63/EU on animal experimentation. The protocol used in this study was approved by the Institutional Ethics Committee of the University of Douala under the ethics approval number 2322 CEI-UDo/07/2020/M delivered on 1 July 2020.
Ovariectomy of Female Rats
Twenty female rats were ovariectomized according to the method described by Cariton [25], and modified by Watcho et al. [26], to avoid cases of pregnancy during copulation tests. Unfortunately, some deaths were observed during this surgery.
Estrus Induction
Estrus was induced in female rats two weeks following the ovariectomy using oestradiol benzoate and progesterone and, the receptivity of each female was verified before testing as described previously [26,27].
Stress Induction
Two types of psychological stress were used in this study: restraint and cold stress. After one week of acclimatisation in the induction room, rats were exposed to each of these stress paradigms for 8 hours/day for 4 weeks. Each day after the induction period for each stress paradigm, the animals were returned to their home cage.
Restraint Stress Induction: Restraint stress was induced in rats according to the protocol of Bardin et al. [28], as described by Haloui et al. [29], with some modifications. Briefly, each rat was placed in a cylindrical plastic tube (diameter: 6.5 cm, height: 21 cm) containing eight ventilation pores (diameter: 0.6 cm), the upper part of which was closed by a wooden plug with four ventilation pores to allow the rat to breathe. The lower part of the tube was closed by another wooden plug with a hole for the rat’s tail. The cylindrical tube was designed to restrict the animal’s movements.
Cold Stress Induction: The modified protocol of Lordi et al. [30], was used to induce cold stress. Briefly, 10 kg of ice was uniformly introduced into a glass cavity (length: 60 cm; thickness: 40 cm and height: 5 cm) and completely covered with a glass sheet. After a latency period of 10 min, necessary for an even temperature distribution on the surface of the glass sheet, the animals were placed separately in a compartmentalised wooden box (length: 20 cm, width: 10 cm and height: 20 cm, containing 10 chambers) placed above the glass sheet in such a way that the soles of the rats’ four legs were in direct contact with the surface covered by the glass sheet. The upper part of this apparatus was covered by a support plate. The ice supply was replaced every 2 h and the temperature in the ice chamber was maintained at 4°C using a thermometer.
Experimental Design
36 rats were randomly divided into 3 groups (n=12) representing unstressed (control), restraint-stressed (R-stress) and cold-stressed (C-stress) animals to minimise statistical bias. At the end of the stress period, half of the animals in each group (n=6) were sacrificed, while the other half were maintained and observed during a 4-week of watch-out period (satellite groups) to verify the possible reversibility of the parameters altered during the stress period. The sample size was estimated to obtain reliable data for better statistical analysis. To avoid confusion of cages and to ensure blinding during manipulation, the different rat cages were coded, arranged in order and kept in the same position throughout the experimental period, and the manipulator was not aware of the codings and names of the three test groups.
Exploration and Anxiety Parameters Evaluation
In this study, exploration and anxiety parameters were assessed using validated experimental procedures (open field and light/dark box). These tests were conducted before and after each induction stress period and after the watch-out period.
Open Field Test: The open field test was performed according to the method described by Solomonow and Tasker [31]. In a wooden open field tank (40×40×40 cm) with the floor divided into 16 equal squares (10×10 cm2) (4 central and 12 peripheral), the rat was placed in the central part of the central zone and its exploration was recorded for 5 min using a camera mounted above the apparatus. The videotape was analyzed to determine the following parameters of exploration (crossing: total number of lines crossed by the animal; sniffing: total number of sniffs) and anxiety (time spent in the central and peripheral areas and number of central entries). The box was cleaned with 95°C alcohol after the passage of each animal to avoid any distraction by odour that could influence the test.
Light/Dark Test: The light/dark test was performed according to the protocol of Solomonow and Tasker [31]. The apparatus consisted of a wooden box (40cm×20 cm×20 cm), containing two compartments: a light compartment representing 2/3 of the box and a dark compartment representing 1/3 of the total box length. A passage 8 cm high and 6.5 cm wide was made between the two compartments. A light bulb was placed above the light chamber. At the beginning of the experiment, a rat was placed in the lighted compartment and recorded for 5 min. The videotape was examined to determine anxiety parameters (time spent in the light compartment, time spent in the dark compartment, and number of entries into the light compartment). As in the open field test, the box was cleaned with 95 % alcohol after each animal’s passage. in the open field test, the box was cleaned with 95° alcohol after the passage of each animal.
Sexual Behaviour Testing Procedure: The sexual behaviour test was performed before and after stress induction and after the watch-out period following the methodology described previously [32]. The following parameters of copulation were recorded in a calm, quiet and dimly lit enclosure: Mount Latency (ML), Intromission Latency (IL), Ejaculation Latency (EL), Mount Frequency (MF), Intromission Frequency (IF), Ejaculation Frequency (EF), Mean Interval of Copulation (MIC), Post-Ejaculatory Interval (PEI). Prior to stress induction, the recording of an IL equal to or greater than 20 minutes (1200 seconds) and an IF equal to zero were criteria for the exclusion of rats, and rats showing these values were excluded from the study and replaced. These criteria were set a priori. No data were excluded during the analyses.
Sacrifice, Sample Collection and Tissue Preparation
At the end of the experiment, each rat in each group was sacrificed by decapitation under diazepam (10 mg/ kg)/ketamine (50 mg/kg) anaesthesia. An intraperitoneal injection of diazepam (10 mg/kg, a muscle relaxant), which is responsible for muscle relaxation, was followed ten minutes later by an intraperitoneal injection of ketamine (50 mg/kg), the anaesthetic itself, which renders nerve endings insensitive. A few minutes after the ketamine injection, the animal was restrained, the neck weights were shaved and an incision was made to cut the jugular vein. The animal was placed supine on a dissecting board. After incision of the skin and then the peritoneum, arteriovenous blood was immediately collected in dry tubes, held at room temperature for sedimentation and centrifuged at 1118 g for 15 min, and the serum obtained was aliquoted into 1.5 ml Eppendorf tubes and stored at -20°C for evaluation of total serum protein, total cholesterol and testosterone levels. Sexual organs (testes, epididymis, vas deferens, prostate, and seminal vesicle), brain and adrenals were removed, cleaned, washed in 0.9% NaCl solution and weighed using a KERN EMB600-2 sensitive balance to determine the relative mass of the organs. Removed testes and epididymis were cleaned, washed in 0.9% saline, crushed and homogenised at 15 and 10% in potassium and sodium buffer, respectively, in an ice bag. The different homogenates were used for the assessment of oxidative stress markers. Part (half) of the right testis was used for daily sperm production and evaluation of its efficiency, and the other half for evaluation of oxidative stress markers, while the left testis was used for histomorphometric evaluations. The right epididymis was used for sperm count, motility and transit and the left for oxidative stress markers. The brain was used for histomorphometric examination of the hippocampal zones.
Evaluation of the Relative Masses of Organs and the Volume of Testes: The absolute masses of the reproductive organs (testes, epididymis, vas deferens, prostate and seminal vesicle) and the adrenal gland obtained behind were used to calculate their respective relative masses using the following formula:
Relative mass (%) = (Absolute mass/Body mass) ×100
The volume of the testes was calculated according to the formula: V (cm3) = (Length) cm × (width)2 cm × 0.52
Biochemical Assays: Total cholesterol and total protein levels were assessed by spectrophotometry (BK-UV-1600 PC) at 505 nm and 540 nm, respectively, using SGM Italia kits. Serum testosterone was assessed using ErbaLisa® kits for testosterone (Erba Mannheim, IME00019, Calbiotech Inc, Cordell Court, El Cajon, CA 92020 USA, sensitivity: 1.16 ng/ml) according to the manufacturer’s instructions, using an Enzyme-Linked ImmunoSorbent Assay (ELISA) and a microplate reader (Microreader V-320). Malondialdehyde (MDA), Nitric Oxide (NO) and reduced glutathione (GSH) levels, and Superoxide Dismutase (SOD) and Catalase (CAT) activities were measured using different protocols [33].
Testicular daily sperm production and epididymal sperm count, mobility and transit evaluation
Testicular daily sperm production and its efficiency: Daily testicular sperm production was assessed according to the modified method described by Robb et al. [34], with some amendments as described previously [32]. Efficient sperm production was obtained by dividing the DSP by the testicular mass.
Epididymal Sperm Mobility Evaluation: Sperm mobility was determined according to the modified protocol of Robb et al. [34], and Ernst [35], as described by Wankeu-Nya et al. [32]. The percentage of motile spermatozoa was calculated using the following formula
% of sperm motility= (motile sperm count / total sperm count) ×100
Epididymal Sperm Count Evaluation: Epididymal sperm count was performed according to the method of Ernst [35], with some modifications [32].
Epididymal Sperm Transit Evaluation: Epididymal sperm count was divided by the DSP to determine the epididymal sperm transit [34].
Histomorphometric Analyses
Histomorphometric examination of testes and brain was performed according to the protocol defined by Chauhan and Dixit [32,36]. The collected microphotographs were used to determine the diameter of the seminiferous tubules, the height of the germinal epithelium in the testes and the number of neurons in Ammon’s horns 1 (CA1) and 3 (CA3) using ImageJ software version 1.50.
Statistical Analysis
Data were expressed as the mean ± SEM. Statistical analyses were performed with GraphPaD Prism software version 5.03. Normality of distribution and homogeneity of variance were assessed using Kolmogorov-Smirnov normality test (with Dallal-Wilkinson-Lillie for p value) and Levene’s tests respectively. The daily and effective sperm productions, sperm mobility, sperm count, epididymal sperm transit, relative mass of organs, volume of testes, biochemical parameters and histomorphometric measurements were analyzed using One-way ANOVA followed by the Bonferroni post-test. Two-way ANOVA followed by the Bonferroni post-test were used to analyze the body mass, exploration, anxiety and sexual behaviour parameters. The p value was considered statistically significant to p<0.05.
RESULTS
Effects of Restraint and Cold Stress on Exploration and Anxiety Parameters, and Body Mass of Rat
No significant difference was observed between the three groups before stress in open field and ligh/ dark parameters, and body mass respectively. However, compared to the control and the period before stress, significant (p<0.05-0.001) decreases in the number of crossing, sniffing, the time spent in the centre area (TSCA), the number of centre entries (NCE), the time spent in the light zone (TSLZ), the number of the light zone entries (NLZE) and body mass, coupled to significant (p<0.05 0.001), increases in the time spent in the peripheral area (TSPA) and the time spent in the dark zone (TSDZ), were observed in animals after 4 weeks of restraint and cold stress respectively. These modifications were more important in restraint stressed animals especially for the number of crossing (62.09 and 58.74% of decreases respectively), the TSCA (95.18 and 94.64% decreases respectively), the TSDZ (56.93 and 68.04% of increases respectively), the TSLZ (88.32 and 90.04% decreases respectively) and the body mass (22.53 and 12.15% decreases respectively). After a watch-out period, no significant variation was recorded for open field and light dark parameters, and body mass compared to the control. But, compared to their respective values after 4 weeks of stress, significant (p<0.01-0.001), increases were recorded for crossing (R-stress, 50.93%), NCE (R-stress: 81.13%; C-stress: 85.03%), TSCA (R-stress: 95.72%; C-stress: 88.37%), TSCA (R-stress: 95.72%; C-stress: 87.08%), TSLZ (R-stress: 89.23%; C-stress: 45.22%) , NLZE (R-stress: 69.96%; C-stress: 41.35%) and body mass (R-stress: 12.46%; C-stress: 5.86%), coupled to significative decreases in the TSPA (R-stress: 15.02%; C-stress: 11.42%) and the TSDZ (R-stress: 68.34%; C-stress: 62.98%) (Table I).
Table I: Effects of restraint and cold stress on exploration and anxiety parameters, and body mass of rat.
|
Open ????ield test |
|||||||||
|
|
Before stress |
After 4 weeks of stress |
After watch-out period |
||||||
|
Parameters |
Control |
R-stress |
C-Stress |
Control |
R-Stress |
C-Stress |
Control |
R-Stress |
C-Stress |
|
Crossing |
83.6 ± 10.24 |
84.83 ±7.70 |
81.5 ± 4.10 |
92.33 ± 5.85 |
35 ± 5.70***γ |
53 ± 4.98*** β |
87.16 ± 5.85 |
71.33 ± 4.40b |
71.5 ± 2.56 |
|
Sniffing |
67.33 ± 10.04 |
65.66 ± 6.43 |
65.66 ± 3.73 |
65.66 ± 5.71 |
19.66 ± 3.73***γ |
32.83 ± 2.78**γ |
63.60 ± 5.71 |
39.16 ± 1.42γ |
39 ± 1.80 |
|
NCE |
12 ± 1.29 |
11.16 ± 0.83 |
11.33 ± 0.84 |
13.16 ± 0,53 |
02 ± 0.36***γ |
01.5 ± 0.22***γ |
11.50 ± 0,66 |
10.6 ± 0.36c |
10.2 ± 0.18c |
|
TSCA (s) |
40 ± 2.69 |
37.33 ± 4.90 |
40.5 ± 3.53 |
41.5 ± 2.91 |
02 ± 0.35***γ |
05 ± 2.44***γ |
39 ± 2.91 |
46.75 ± 5.08c |
38.70 ± 6.67αc |
|
TSPA (s) |
260 ± 2.69 |
262.66 ± 4.90 |
259.5 ± 3.53 |
258.5 ± 2.91 |
298 ± 0.35***γ |
295 ± 2.44***γ |
261 ± 2.91 |
253.25 ± 7.47c |
261.30± 8.54c |
|
Light-dark test |
|||||||||
|
TSDZ (s) |
89.83 ± 26.48 |
89.16 ± 30.78 |
108.66 ± 18.93 |
120.16 ± 4.40 |
279 ± 2.76***γ |
250.33± 12.68*** γ |
122.83± 4.45 |
888.33 ± 19.33c |
92.66 ± 17.01c |
|
TSLZ (s) |
210.16 ± 26.48 |
210.83 ± 30.78 |
191.33 ± 18.93 |
179.83 ± 4.40 |
21 ± 2.76***γ |
49.66 ± 12.68*** γ |
177.16 ± 4.45 |
211.67±9.46c |
190.66 ± 13.52c |
|
NLZE |
4.33 ± 0.21 |
3.83 ± 0.16 |
3.83 ± 0.40 |
3.66 ± 0.22 |
1.50 ±0.22**γ |
2.50 ± 0.22 |
4± 0.25 |
5.16 ± 0.40c |
44.16 ± 0.40b |
|
Body mass (g) |
|||||||||
|
|
240 ± 5.25 |
234.5 ± 3.19 |
234.33 ± 3.11 |
265.92 ± 7.84 α |
206 ± 4.88*** α |
228.21 ± 2.03*** |
272.83 ± 6.89 β |
235.33± 6.65b |
242.42 ± 4.68b |
Effects of Restraint and Cold Stress on Sexual Behaviour Parameters
No significant variation (p>0.05), in the different copulatory parameters was recorded before the stress period between stressed and control groups. However, compared to the control and the period before stress respectively, significant increases (p<0.05-0.001), in the mount, intromission, ejaculation latencies and the post-ejaculatory interval (ML, IL, EL and PEI), coupled to significant decreases (p<0.05-0.001) in the mount, intromission, ejaculation frequencies and the mean interval of copulation (MF, IF, EF and MIC), were registered in the two stressed animal groups after four weeks of stress. These alterations were more significative (p<0.05-0.001), in cold stressed animals compared to their restraint stressed counterparts, especially for the ML (91.76 and 95.24% of increases), IL (93.57 and 94.60% of increases), MF (55.71 and 47.97% of decreases), and IF (62.43 and 54.97% of decreases). Moreover, no significant difference in the sexual behaviour parameters was recorded in both stressed groups after a watch-out period of 4 weeks in comparison to the control; however, compared to their respective values after stress, significant (p<0.001) decreases in the ML (5-19 times), IL (5-12 times) and EL (0.5 time), associated to increases in the MF, IF (p?0.05), EF (p<0.05; 1.9 time for R-stress) and the MIC (p<0.001; 1.40 time for C-stress) were noticed (Table 2).
Table 2: Variation of sexual behaviour parameters in chronic restraint and cold-stressed rat.
|
|
Before stress |
After 4 weeks of stress |
After watch-out period |
||||||||
|
|
Control |
R-Stress |
C-Stress |
Control |
R-Stress |
C-Stress |
Control |
R-Stress |
C-Stress |
||
|
ML |
32.67 ± 1.38 |
39 ± 20.17 |
36.12 ± 11.47 |
62.6 ± 13.64 |
197.1 ± 9.82***γ |
759.72 ± 21.72***γ |
36.33 ± 12.76 |
38.75 ± 5.69c |
38.50 ± 44.40c |
||
|
IL |
62.83 ± 16.83 |
59 ± 3.18 |
52.67± 1.91 |
62.77 ± 12.29 |
274.30 ± 46.50***γ |
975.67 ± 52.85***γ |
78.60 ± 12.85 |
51.25 ± 8.03c |
78.17 ± 42.97c |
||
|
EL |
765.8 ± 62.57 |
703.5 ± 101.86 |
502.88 ± 21.64 |
884.67 ± 98.67 |
1395.75 ± 91.75**β |
1278,00 ± 44,28 γ |
698 ± 66.70 |
568.17± 205.44c |
519.83± 148.80c |
||
|
PEI |
398.28 ± 63.27 |
422.83 ± 33.56 |
481.5± 44.40 |
514.97 ± 36.07 |
481.39 ± 25.69 |
482.50 ± 7.04 |
523.14 ± 36.54 |
675.60 ± 37.21 |
554.22 ± 136.19 |
||
|
MF |
72.50 ± 2.88 |
70.67 ± 6.28 |
82 ± 2,30 |
96.33 ± 15.21 |
47.80 ± 4.63** |
42.67 ± 10,04** |
84 ± 10.85 |
68.75 ± 5.52 |
61.67 ± 9.60 |
||
|
IF |
45.17 ± 0.48 |
53.17 ± 5.77 |
59.5 ± 2.99 |
71.33 ± 10.47 |
32.17 ± 1.44* |
26.80 ± 7.82*** α |
66.33 ± 9.12 |
51.17 ± 3.52 |
46.50 ± 7.97 |
||
|
EF |
3.17 ± 0.47 |
3.5 ± 0.61 |
4 ± 00 |
3.50 ± 0.42 |
2.50 ± 0.42 |
1.83 ± 0.47 α |
4 ± 0.25 |
4.67 ± 0.42a |
5.60 ± 0.20* |
||
|
MCI |
3324.5 ± 53.08 |
3459.5 ± 43.81 |
3431 ± 68.76 |
3418.00 ± 61.02 |
3201.17 ± 140.67*** |
2320.40 ± 296,99 γ |
3331.83 ± 99.40 |
3430.00 ± 50.86 |
3258.33± 55.21c |
||
Effects of Restraint and Cold Stress on the Relative Sexual Organs and Adrenal Gland Masses, and the Volume of Testes
According to Table 3 and compared to control,
Table 3: Variation of the relative sexual organs and adrenal gland masses, and the volume of testes in restraint and cold-stressed rats.
|
|
After 4 weeks of stress |
After watch-out period |
||||
|
Organs |
Control |
R-Stress |
C-Stress |
Control |
R-Stress |
C-Stress |
|
Relative mass (g/100 g of body weight) |
|
|
|
|
|
|
|
Testes |
0.51 ± 0.01 |
0.51 ± 0.01 |
0.56 ± 0.02 |
0.49 ± 0.02 |
0.48 ± 0.05 |
0.53 ± 0.01 |
|
Epididymis |
0.19 ± 0.01 |
0.19 ± 0.02 |
0.19 ± 0.01 |
0.18 ± 0.01 |
0.17 ± 0.01 |
0.17 ± 0.01 |
|
Vas deferent |
0.04 ± 0.01 |
0.07 ± 0.01 |
0.08 ± 0.00** |
0.04 ± 0.01 |
0.08 ± 0.01** |
0.13 ± 0.01***c |
|
Seminal vesicle |
0.29 ± 0.03 |
0.44 ± 0.05 |
0.36 ± 0.03 |
0.27 ± 0.04 |
0.34 ± 0.05 |
0.41 ± 0.04 |
|
Prostate |
0.13 ± 0.00 |
0.14 ± 0.02 |
0.17 ± 0.01 |
0.13 ± 0.01 |
0.13 ± 0.01 |
0.15 ± 0.01 |
|
Adrenal gland |
0.008 ± 0.00 |
0.018 ± 0.00*** |
0.012 ± 0.00** |
0.008 ± 0.00 |
0.011 ± 0.00*c |
0.009 ± 0.00 |
|
Volume of testes (cm3) |
1341.30 ± 61.33 |
639.34 ± 60.55*** |
818.90 ± 78.55*** |
1100.33 ± 10.92c |
761.49 ± 48.54 |
809.37 ± 36.28 |
no significant variation was observed in the relative mass of the sexual organs after the stress induction in both stressed animal groups, except in the relative mass of the vas deferent in cold stressed rats where 62.50% of increase was noticed. In addition, the relative mass of the adrenal gland increased 1.43-2.43 times (p?0.01-0.001), in cold and restraint-stressed rats respectively compared to control, while the volume of testes decreased 1.64 2.1 times in the same groups. After a watch-out period significant increases (p<0.05-0.001), were recorded in the vas deferent (R-stress: 62.50%; C-stress: 76.92 and 38.46%), and adrenal gland (R-stress: 18.18% and 35.29%) relative masses, compared to the control and the period after stress respectively.
Effects of Restraint and Cold Stress on Biochemical Parameters in Rats
After 4 weeks of stress and compared to the control group, significant increases (p<0.05-0.001), in malondialdehyde (MDA) levels coupled to decreases in catalase (CAT) and Superoxide Dismutase (SOD) activities, Nitric Oxide (NO) reduced glutathione (GSH), serum total proteins, total cholesterol and testosterone levels were obtained in animals submitted to restraint and cold stress. However, values were more important in cold-stressed animals for testicular MDA (p<0.001; 55.88%), epididymal NO (p<0.001; 60%), epididymal SOD (p<0.001; 42.86%) and epididymal GSH (p<0.001, 99.76%), while data were more significative in restraint-stressed rats for testicular CAT (p<0.001; 99.75%), serum total proteins (p<0.05; 37.14%), serum total cholesterol (p?0.05; 40.43%) and serum testosterone (p<0.001; 71.32%). Moreover, after a watch-out period, significative increases (p<0.05-0.001) in testicular and epididymal MDA and serum total cholesterol levels associated with decreases (p<0.05 - 0.001) in SOD and CAT activities, GSH, NO, serum total proteins and testosterone levels were noticed in the two stressed groups in comparison to control animals. Compared to the period after stress, no significant variation was registered in all groups after a watch-out period, except in serum total cholesterol where a 58.31% was observed (Table 4).
Table 4: Evolution of biochemical parameters in serum, testes and epididymis after 4 weeks of restraint and cold stress in rats.
|
|
After 4 weeks of stress |
After a watch-out period |
||||
|
Parameters |
Control |
R-Stress |
C-Stress |
Control |
R-Stress |
C-Stress |
|
MDA |
|
|
|
|
|
|
|
Testes |
0.45 ± 0.01 |
0.77 ± 0.00** |
1.05 ± 0.00*** |
0.47 ± 0.01 |
0.71 ± 0.04* |
0.89 ± 0.02* |
|
epididymis |
3.34 ± 0.27 |
4.81 ± 0.32 |
4.49 ± 0.31 |
3.31 ± 0.2 |
4.01 ± 0.58 |
4.62 ± 0.32* |
|
NO |
|
|
|
|
|
|
|
Testes |
0.001 ± 0.00 |
0.0002 ± 0.00*** |
0.0005 ± 0.00*** |
0.001 ± 0.00 |
0.0006 ± 0.00** |
0.0009 ± 0.00* |
|
Epididymis |
0.003 ± 0.00 |
0.0003 ± 0.00*** |
0.0008 ± 0.00*** |
0.002 ± 0.00 |
0.0006 ± 0.00*** |
0.0007 ± 0.00** |
|
SOD |
|
|
|
|
|
|
|
Testes |
0.03 ± 0.00 |
0.02 ± 0.00 |
0.02 ± 0.00 |
0.04 ± 0.00 |
0.03 ± 0.00 |
0.02 ± 0.00* |
|
Epididymis |
0.14 ± 0.00 |
0.09 ± 0.00*** |
0.08 ± 0.00*** |
0.15 ± 0.00 |
0.08 ± 0.00*** |
0.07 ± 0.00*** |
|
CAT |
|
|
|
|
|
|
|
Testes |
0.49 ± 0.01 |
0.12 ± 0.00*** |
0.17 ± 0.00*** |
0.52 ± 0.01 |
0.11 ± 0.05*** |
0.22 ± 0.00*** |
|
Epididymis |
0.73 ± 0.11 |
0.27 ± 0.05* |
0.45 ± 0.06 |
0.77 ± 0.01 |
0.35 ± 0.12** |
0.39 ± 0.06* |
|
GSH |
|
|
|
|
|
|
|
Testes |
0.18 ± 0.00 |
0.10 ± 0.02 |
0.13 ± 0.00 |
0.17 ± 0.00 |
0.09 ± 0.00 |
0.14 ± 0.00 |
|
Epididymis |
0.54 ± 0.08 |
0.15 ± 0.03*** |
0.13 ± 0.02*** |
0.63 ± 0.01 |
0.16 ± 0.04*** |
0.11 ± 0.03*** |
|
Serum total proteins |
199.62 ± 16.27 |
125.57 ± 18.16* |
143.56 ± 16.96 |
203.12 ± 2.30 |
145.93 ± 14.56 |
151.64 ± 6.81 |
|
Serum total cholesterol |
61.46 ± 8.24 |
36.61 ± 6.76 |
48.78 ± 0.26 |
68.07 ± 0.26 |
87.82 ± 1.05c |
113.35 ± 5.54*** |
|
Serum testosterone |
6.38 ± 0.21 |
1.83 ± 0.82*** |
3.10 ± 0.27*** |
6.90 ± 0.03 |
2.38 ± 0.59*** |
4.55 ± 0.05* |
Effects of Restraint and Cold Stress on Testicular and Epididymal Sperm Parameters
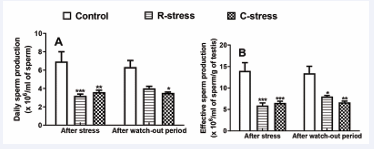
Effects on Testicular Daily and Effective Sperm Production: Figure 1
Figure 1 : Variation in daily (A) and effective (B) sperm production in rats after 4 weeks of stress and after a watch-out period. Each bar represents the mean ± SEM; n=6; *: p<0.05, **: p<0.01, ***: p<0.001, significant difference compared to the control; control: neutral control group. R-stress: restraint stress group. C-stress: cold stress group; One way and two way (repeated measures) ANOVA followed by Bonferroni post-test.
reveals significant (p<0.01-0.001) decreases of 53.89% and 58.12% in daily sperm production, and 57.69% and 53.32% in effective sperm production after 4 weeks of stress in animals submitted respectively to restraint and cold stress compared to control. In addition, compared to the control, a significant (p<0.01-0.001), drop in daily and effective sperm production was noticed in animals after the watch-out period, but no significative variation was registered during this period in stressed groups compared to the period of stress.
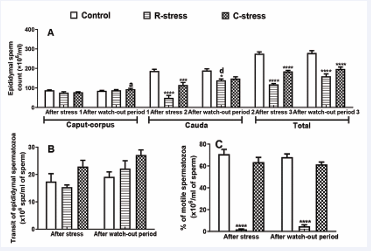
Effects on Epididymal Sperm Mobility, Sperm Count and Transit: Results obtained after 4 weeks of stress show that, compared to the control, 95.63% (p?0.001;R-stress) and 10.49% (p?0.05; C-stress) of decreases in the percentage of sperm mobility were registered in the two stressed groups. Moreover, after a watch-out period 93.15% and 17.93% of decreases in this parameter were recorded in stressed rats compared to the control (Figure 2A). According to the Figure 2B, 11.60% of decrease coupled to 23.94% of increase in the epididymal sperm transit were noticed in R-stress and C-stress respectively compared to the control.
Figure 2 : Variation in the sperm count (A), sperm mobility (B) and sperm transit in the epididymis of rats after 4 weeks of stress and after a watch-out period.
Each bar represents the mean ± SEM; n=6; *: p<0.05, **: p<0.01, ***: p<0.001, significant difference compared to the control; a: p<0.05; b: p<0.01, c: p<0.001, significant difference compared to the period of stress. R-stress: restraint stress group; C-stress: cold stress group. One way and two way (repeated measures) ANOVA followed by Bonferroni post-test.
After a watch-out period, 13.69% and 29.49% of increases were observed in both stressed groups compared to their control. No significative variation in these two parameters was observed in stressed groups after a watch-out period in comparison to the period after stress. After stress in both stressed groups, significant decreases were observed in the caput-corpus (p?0.05; 14.28%; 11.78%), cauda (p?0.001; 73.80%; 38.45%) and total (p?0.001; 57.13%; 32.91%) epididymal sperm count compared to their respective control. Moreover, after the watch-out period, significant increases were recorded especially in the cauda (R-stress: p?0.001, 26.89%; C-stress: p?0.05, 21.99%) and the total (R-stress: p?0.001, 42.67%; C-stress: p?0.001, 29.24%) epididymis. Compared to the period after stress, this parameter increased in the caput-corpus (C-stress: p?0.05; 16.75%) and the cauda (R-stress: p?0.001; 64.54%).
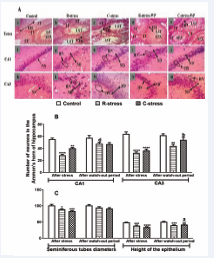
Effects of Restraint and Cold Stress on the Structure of the Testes and Hippocampus: In the testes of the two stressed animal groups, alterations in the seminiferous tubules (characterized by the reduction of sperm density in the lumen, the decreases in its diameter and epithelial height (p?0.001)) coupled to the hypertrophy of the interstitial tissue with damages of Leydig cells were observed after the stress and after the watch-out period respectively compared to the control. After the watch-out period and compared to the period after stress, alleviations in the altered parameters were noticed, marked by increases in the seminiferous diameter (p?0.05) and the height of its epithelium (p?0.05; 10.77%).
In the hippocampus, compared to the control presenting normal neuronal structure, the presence of pycnotic neurons with hyperchromatic nucleus in the Ammon’s horns 1 and 3 (CA1 and CA3), and the significant (p?0.001) neurodegeneration of these cells (CA1-R-stress: 48.20% and 17.11%; CA1-C-stress: 27.11% and 18.26%; CA3-R stress: 49.34% and 28.83%; CA3-C-stress: 43.11% and 12.40%) were observed in the two stressed groups after the stress and the watch-out period respectively. These alterations were more important in the R-stress group compared to the C-stress one. After the watch-out period and compared to the period after stress, significative increases in CA1 (R-stress: p?0.001; 39.86%) and CA3 (C-stress: p?0.01; 31.60%) (Figure 3).
Figure 3 : Microphotographs (X100, 5 µm thickness, H&E Coloring) (A) of the testes (a-e) and Ammon’s horn 1 (CA1) (f-j) and 3 (CA3) (k-o) and variation in the diameter of the seminiferous tubules and the height of the epithelium (C), and in the number of cells in CA1 and CA3 (A) of the hippocampus in rats after 4 weeks of stress and after a watch-out period.
R-stress: restraint stress group. C-stress: cold stress group. R-stress-WP: restraint stress group after a watch-out period. C-stress-WP: cold stress group after watch-out period. CA1: Ammon’s horn 1; CA3: Ammon’s horn 3. ST: Seminiferous tubule. IT: Interstitial tissue. LTS: lumen of the seminiferous tubule. SPZ: spermatozoa. ND: neurodegeneration; HN: hyperchromatic neuron. NN: Normal neuron. EH: Epithelium height. H&E: Hematosilin and eosin.
DISCUSSION
It’s well known that exposure to chronic stress can have detrimental effects on the brain and predispose to emotional and reproductive disorders [24]. However, studies demonstrating the precise neuroendocrine mechanisms involved in the development of anxiety and reproductive disorders are limited. Furthermore, the effects of many types of stressors, in particular restraint and cold stress, as well as the effects of a watch-out period after the induction of chronic stress have not yet been examined. The aim of the present study was, firstly, to investigate the effects of two models of psychological stress (restraint and cold stress) on neurobiological and reproductive parameters, and, secondly, to investigate the effects of two models of psychological stress (restraint and cold stress) on neurobiological and reproductive parameters. No significant differences were observed between the three pre-stress groups in exploratory and anxiety parameters or body mass. However, compared to the control and pre-stress periods, significant decreases in the number of crossings, sniffing, Time Spent in the Central Area (TSCA), Number of Central Entries (NCE), Time Spent in the Light Zone (TSLZ), Number of Light Zone Entries (NLZE) and body mass were observed in restrained and cold-stressed animals. Significant increases in Time Spent in the Peripheral Area (TSPA), Time Spent in the Dark Zone (TSDZ) and adrenal mass were also observed in cold and restraint stressed animals compared to the pre-stress period and controls. These changes were more pronounced in the restrained rats. These impairments observed above are characteristic of decreases in exploratory and locomotor activity associated with increases in anxiety parameters induced by the two stress models reported by Sarkar [37]. Similar findings have been reported by other authors [38,39], and could be attributed to the inhibition of dopamine synthesis and utilisation in the central nervous system, particularly in the dopaminergic system (frontal cortex, nucleus accumbens of the hippocampus and neostriatum) following high production of glucocorticoids and catecholamines by the Hypothalamic-Pituitary-Adrenal (HPA) axis. Various stress manipulations are known to induce changes in motor activity and dopaminergic pathways in animals; high sensitivity to stress is thought to be involved in the activation of goal-directed behaviour, whereas its inhibition may lead to emotional indifference, lack of initiative and a decrease in locomotor activity [40 42]. In the present study, changes were more pronounced in restraint stressed animals compared to cold stressed animals, suggesting that restraint stress may be more deleterious than cold stress, as reported by Xu et al. [43]. These results support the findings of Heinz et al. [42], and confirm that when an animal is anxious, its exploratory activity decreases with a preference for the dark zone. Moreover, the increase in exploratory parameters and the decrease in anxiety recorded in the satellite groups after the wakefulness period are in line with these results and, moreover, indicate a possible restoration of these parameters in the absence of the stressor during this period; that is, the non-activation of the stress axis and a reactivation of dopamine synthesis [44]. According to Selye [5], variations in body and adrenal mass are indicators of the state of anxiety in animals. Therefore, the significant weight loss and increase in relative adrenal mass observed in the present study in stressed groups of animals supports his hypothesis and could be attributed to hyperactivation of the HPA axis and the sympathetic-adrenal system [31]. The lower serum concentrations of total cholesterol and total protein, particularly in restrained animals, may also explain the results obtained here. During stress, cholesterol (precursor of steroid hormones) is normally used for the synthesis of glucocorticoids [45], which inhibit protein synthesis and prevent the transport of amino acids in the muscles [43]. The progressive increase in total cholesterol and total protein levels observed in the stressed animals after the watch-out period could be responsible for their weight gain, due to the cessation of stimulation of the HPA axis and production of stress hormones, as previously demonstrated.
Chronic exposure of animals to immobilisation and cold has been shown to induce activation of the Sympatho-Adrenomedullary System (SAS) resulting in the release of stress hormones [46]. Thus, restraint stress activates the HPA and increases the release of the hormone corticoliberin (CRH) from the paraventricular nucleus of the hypothalamus, which causes the secretion of adrenocorticotrophin (ACTH) from the anterior pituitary. This allows the release of glucocorticoids by the adrenal gland [47]. Cold also stimulates the peripheral thermoreceptors, generating sensitive nerve impulses that are transmitted to the preoptic area of the anterior hypothalamus (an area sensitive to temperature changes), allowing the production of CRF, which leads to the release of glucocorticoids from the adrenal gland [48].
The hippocampus is a key brain area involved in the regulation of the stress response and is known to provide negative feedback to the hypothalamic-pituitary adrenal (HPA) axis [49]. Therefore, exposure to various stressors may alter hippocampal function and structure. In the present study, histomorphometric examination of hippocampal regions showed a decrease in neuronal density and hyperchromatic nuclei in the Ammon’s horn 1 and 3 (CA1 and CA3) regions of the hippocampus. These findings are similar to those reported by Beppe et al. [50], in mice exposed to chronic mild stress, oxidative stress induced inhibition of neurogenesis/stem cell proliferation and loss of dendritic spines (thus reducing nerve connections) as a result of elevated glucocorticoid levels, leading to a reduction in the production of new neurons [12,51], and ultimately to hippocampal atrophy and cognitive dysfunction [13,52,53].
The evaluation of sexual behavioural parameters in animals subjected to restraint and cold stress showed a decrease in copulatory activity, characterized by a decrease in the frequency of mounts, intromissions and ejaculations, as well as the mean interval of copulation, coupled with a significant increase in mounts, intromissions, ejaculatory latencies and post-ejaculatory interval compared to the control. The decrease in intromission frequency observed in stressed animals suggests that erectile activity was inhibited, probably due to activation of adrenergic tone during stress [54,55]. In stressful situations, adrenergic stimulation activates sympathetic nerve endings, causing contraction of the trabecular smooth muscle fibres of the penis and its detumescence [56,57]. Also, given that androgens are involved in sexual behaviour, this stress related decrease in sexual performance could be due to a decrease in testosterone levels observed in this study. Testosterone exerts its effects on sexual behaviour by binding to androgen receptors in the brain at the level of the preoptic area of the hypothalamus, the limbic system and the cerebral cortex [58,59]. During chronic stress, the excessive secretion of glucocorticoids alters the secretion of pituitary gonadotropins, which is responsible for hypogonadotropic hypogonadism, causing a decrease in steroidogenesis [60,61]. In addition, in erectile function, androgens stimulate the expression of the neuronal isoform of nitric oxide synthase (nNOS) and modulate the activity of phosphodiesterase type 5 during erection. As stress has been shown to decrease nNOS [62], therefore, the decrease in testicular and epididymal NO levels observed in stressed animals could explain the decrease in sexual behaviour observed in the latter. Furthermore, the return of sexual behaviour parameters after the observation period could be due to an end of stimulation of the HPA axis and hence inhibition of muscle tone. In this study, significant decreases in testicular volume were observed in the stressed animals, particularly in the restrained animals. These decreases could be explained by the reduction in the levels of gonadostimulins FSH and LH observed in a stressful situation and subsequently, causing testicular atrophy in addition to the reduction in gonadal activity [63,64].
Analysis of testicular sperm parameters after 4 weeks of stress revealed a significant decrease in daily and effective sperm production in stressed animals. As mentioned previously, during chronic stress, the excessive secretion of corticosteroids alters the secretion of pituitary gonadotropins and may be responsible for the decrease in spermatogenesis observed here [65,66]. In addition, the decrease in serum testosterone levels associated with the reduction in recorded testicular volume may also explain this reduction in sperm production. Observations of epididymal sperm parameters showed a significant decrease in sperm count and motility in stressed animals, as well as an increase in transit time after 4 weeks of stress compared to controls. These changes in sperm count, motility and transit could be due to the production of activated oxygen species (AOS) and the subsequent rapid loss of intracellular ATP [67,68]. These results are in line with those of Sabeti et al [69], who stated that oxygen species activated in response to exogenous or endogenous stress may have deleterious effects on epididymal sperm count, motility and transit. In addition, excessive production of free radicals could lead to peroxidation reactions in spermatozoa due to their high content of polyunsaturated fatty acids. The elevated levels of MDA observed in the testes and epididymis thus confirm this hypothesis and corroborate the findings of other authors [70,71]. Furthermore, the decrease in testicular and epididymal superoxide dismutase (SOD) and catalase (CAT) activities and GSH levels observed in this study are also markers of oxidative stress. Ayad et al. [72], reported that chronic psychological stress can induce damage to cell membranes, proteins and DNA, leading to alterations in the spermatogenic process and thus in sperm parameters. The lack of return of these parameters after the observation period correlates with the low testosterone levels, sperm motility and long sperm transit times observed in the animals and may indicate that stress-altered sperm parameters require more time to normalise, even after the stressor is removed. These results are consistent with the findings of previous authors who suggested that normalisation of the HPA axis may not be automatic after removal of stressors and that other factors may be involved, leading to delayed recovery of higher centres [73]. To confirm the changes in testosterone and sperm production induced by chronic stress, histomorphometric studies of the testes of stressed animals were performed, and the results indicated a decrease in sperm density in the lumen of the seminiferous tubules of the testes, in the diameter of the seminiferous tubules and in the height of the germinal epithelium, coupled with hypertrophy of the interstitial tissue. These results could also be attributed to the oxidative stress induced by chronic psychological stress. It is known that in a situation of chronic stress, the high level of glucocorticoids inhibits the production of testosterone by the Leydig cells, causing disorganisation of the germ cells in the seminiferous tubules and hypertrophy of the interstitial tissue [74]. Chronic stress can also lead to the production of substances that can activate glutamate NMDA receptors, resulting in excitotoxicity and consequently oxidative stress [75]. After the observation period, these results showed a progressive restoration of the testicular structures due to the absence of stressors and therefore a decrease in glucocorticoid secretion. The fact that serum corticosterone levels were not determined in this study is a limitation of this work.
CONCLUSION
In conclusion, the results of this study showed that the two stress models used in this work caused a decrease in exploratory activity and an increase in anxiety levels, a decrease in copulatory and fertility parameters, and damage to testicular and hippocampal histology and function, particularly in restraint stressed rats. The results observed after the observation period indicated reversible and non-reversible impairments in neuroreproductive parameters. These results show that restraint and cold stress damage function and structure in different ways, suggesting that the best treatment for neuroreproductive changes in stressed patients may depend on the type of parameter, type and duration of stress.
ACKNOWLEDGEMENTS
The authors are grateful to the Alexander von Humboldt-Foundation for providing laboratory material and equipment to one of the authors of this work, Pr. Dongmo Alain Bertrand. The authors are also grateful to Dr. Fifen Rodrigue and Pr. Dzeufiet Djomeni Paul Désiré of the Laboratory of Animal Biology and Physiology at the University of Yaoundé I for the preparation and interpretation of the histological sections.
ADDITIONAL INFORMATION AND DATA AVAILABILITY
The protocol used in this study and the datasets analysed in this study are available on request from the corresponding author.
AUTHORSHIP CONTRIBUTIONS STATEMENT
WNM and SFF participated in the study’s conceptualization, data curation and formal analysis. SFF, GBM, KTI, DON and HTD carried out the investigations and methodology. WNM was responsible for project administration and WNM and SFF produced the study resources and software. WNM, SFF, TMC, NMI, WKV, BZC and ASB were involved in the validation, visualization and writing of original draft. WNM, MFP DAB, MLD and WP contributed for the supervision, writing - review & editing of the manuscript.
REFERENCES
- Vander Borght M, Wyns C. Fertility and infertility: Definition andepidemiology. Clin Biochem. 2018; 62: 2-10.
- Fainberg J, Kashanian JA. Recent advances in understanding and managing male infertility. F1000Res. 2019; 8: F1000 Faculty Rev- 670.
- Menkveld R, Van Zyl JA, Kotze TJ, Joubert G. Possible changes in male fertility over a 15-year period. Arch Androl. 1986; 17: 143-144.
- Okonofua FE, Ntoimo LFC, Omonkhua A, Ayodeji O, Olafusi C, Unuabonah E, et al. Causes and Risk Factors for Male Infertility: A Scoping Review of Published Studies. Int J Gen Med. 2022; 15: 5985- 5997.
- SELYE H. Stress and the general adaptation syndrome. Br Med J. 1950; 1: 1383-1392.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009; 5: 374-381.
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: Acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001; 14: 1143-1152.
- Ross JA, Van Bockstaele EJ. The locus coeruleus- norepinephrine system in stress and arousal: unraveling historical, current, and future perspectives. Front Psychiatr. 2021; 11: 601519.
- Chrousos GP. Regulation and dysregulation of the hypothalamic- pituitary-adrenal axis. The corticotropin-releasing hormone perspective. Endocrinol Metab Clin North Am. 1992; 21: 833-858.
- Chand D, Lovejoy DA. Stress and reproduction: Controversies and challenges. Gen Comp Endocrinol. 2011; 171: 253-257.
- Wu JX, Lin S, Kong SB. Psychological stress and functional endometrial disorders: Update of mechanism insights. Front Endocrinol (Lausanne). 2021; 12:690255.
- Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One. 2012; 7: e30481.
- McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016; 41: 3-23.
- Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PLoS One. 2011; 6: e18712.
- Züst MA, Colella P, Reber TP, Vuilleumier P, Hauf M, Ruch S, et al. Hippocampus is place of interaction between unconscious and conscious memories. PLoS One. 2015; 10: e0122459.
- Whirledge S, Cidlowski JA. Glucocorticoids and reproduction: Traffic control on the road to reproduction. Trends Endocrinol Metab. 2017; 28: 399-415.
- Lei A, You H, Luo B. The associations between infertility-related stress, family adaptability and family cohesion in infertile couples. Sci Rep. 2021; 11: 24220.
- Bräuner EV, Nordkap L, Priskorn L, Hansen ÅM, Bang AK, Holmboe SA, et al. Psychological stress, stressful life events, male factor infertility, and testicular function: a cross-sectional study. Fertil Steril. 2020; 113: 865-875.
- Joseph DN, Whirledge S. Stress and the HPA Axis: Balancing Homeostasis and Fertility. Int J Mol Sci. 2017; 18: 2224.
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002; 7: 254-275.
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002; 53: 865- 871.
- Kulkarni MP, Juvekar AR. Attenuation of Acute and Chronic Restraint Stress-induced Perturbations in Experimental Animals by Nelumbo nucifera Gaertn. Indian J Pharm Sci. 2008; 70: 327-332.
- Prabsattroo T, Wattanathorn J, Iamsaard S, Somsapt P, Sritragool O, Thukhummee W, et al. Moringa oleifera extract enhances sexual performance in stressed rats. J Zhejiang Univ Sci B. 2015; 16: 179- 190.
- Ciechanowska M, ?apot M, Antkowiak B, Mateusiak K, Paruszewska E, Malewski T, et al. Effect of short-term and prolonged stress on the biosynthesis of Gonadotropin-Releasing Hormone (GnRH) and GnRH Receptor (GnRHR) in the hypothalamus and GnRHR in the pituitary of ewes during various physiological states. Anim Reprod Sci. 2016; 174: 65-72.
- Cariton AE. Experimental surgery of the genital system. In: Gay,W.I. and Heavener JE (ed) Methods of animal experimentation: Research surgery and care of the research animal; Part B Chirurgical approaches to organ systems. Orlando: Academic Press, 1986; 191.
- Watcho P, Wankeu-Nya M, Nguelefack TB. Pro-sexual effects of Dracaena arborea (wild) link (dracaenaceae) in sexually experienced male rats. Pharmacologyonline 2007; 1: 400-419.
- Wankeu-Nya M, Watcho P, Nguelefack TB, Carro-Juarez M, Tapondjou L, Kamanyi A. Effects of dracaena arborea (Dracaenaceae) on sexual dysfunction in 4 weeks hyperglycemic male rats. Asian Pac J Trop Med. 2014; 7: 609-619.
- Bardin L, Malfetes N, Newman-Tancredi A, Depoortère R. Chronic restraint stress induces mechanical and cold allodynia, and enhances inflammatory pain in rat: Relevance to human stress-associated painful pathologies. Behav Brain Res. 2009; 205: 360-366.
- Haloui M, Tahraoui A, Bououza F. Effects of chronic restraint stress on energetic metabolism and the evolution of depression, evaluated in the Open Field test in female wistar rat. 2014; 5: 1-7.
- Lordi B, Patin V, Protais P, Mellier D, Caston J. Chronic stress in pregnant rats: effects on growth rate, anxiety and memory capabilities of the offspring. Int J Psychophysiol. 2000; 37: 195-205.
- Solomonow J, Tasker JG. Anxiety behaviour induced in mice by acute stress. Tulane Undergrad Res J. 2015; 14-19.
- Wankeu-Nya M, Djeumeni ON, Nde Z, Tchamadeu MC, Kengne TI, Hatho TDH, et al. Aphrodisiac and androgenic effects of the aqueous extract of the roots of Vepris afzelii on cyproterone acetate-induced hypogonadism in rat. Int J Impot Res. 2025; 37: 293-302.
- Cand F, Verdetti J. Superoxide dismutase, glutathione peroxidase, catalase, and lipid peroxidation in the major organs of the aging rats. Free Radic Biol Med. 1989; 7: 59-63.
- Robb GW, Amann RP, Killian GJ. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil. 1978; 54: 103-107.
- Ernst E. A method for evaluation of epididymal sperm count and motility in the rat. Scand J Lab Anim Sci. 1989; 16: 67-71.
- Chauhan N, Dixit V. Spermatogenic activity of rhizomes of Curculigo orchioides Gaertn in male rats. Int J Appl Res Nat Prod. 2008; 1: 26- 31.
- Himanshu Dharmila, Sarkar D Nutan. A Review of Behavioral Tests to Evaluate Different Types of Anxiety and Anti-anxiety Effects. Clin Psychopharmacol Neurosci. 2020; 18: 341-351.
- Bloomfield MA, McCutcheon RA, Kempton M, Freeman TP, Howes O. The effects of psychosocial stress on dopaminergic function and the acute stress response. Elife. 2019; 8: e46797.
- Baik JH. Stress and the dopaminergic reward system. Exp Mol Med. 2020; 52: 1879-1890.
- Quaedflieg CWEM, Stoffregen H, Sebalo I, Smeets T. Stress-induced impairment in goal-directed instrumental behaviour is moderated by baseline working memory. Neurobiol Learn Mem. 2019; 158: 42-49.
- van Ruitenbeek P, Quaedflieg CW, Hernaus D, Hartogsveld B, Smeets T. Dopaminergic and noradrenergic modulation of stress- induced alterations in brain activation associated with goal-directed behaviour. J Psychopharmacol. 2021; 35: 1449-1463.
- Heinz DE, Schöttle VA, Nemcova P, Binder FP, Ebert T, Domschke K, et al. Exploratory drive, fear, and anxiety are dissociable and independent components in foraging mice. Transl Psychiatry. 2021; 11-318.
- Xu WJ, Guo K, Shi JL, Guo CT, Xu JL, Zheng R, et al. glucocorticoid regulates the synthesis of porcine muscle protein through m6a modified amino acid transporter slc7a7. Int J Mol Sci. 2022; 23-661.
- Howes OD, McCutcheon R, Owen MJ, Murray RM. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol Psychiatry. 2017; 81: 9-20.
- Hoekstra M, van der Sluis RJ, Van Eck M, Van Berkel TJ. Adrenal- specific scavenger receptor BI deficiency induces glucocorticoid insufficiency and lowers plasma very-low-density and low-density lipoprotein levels in mice. Arterioscler Thromb Vasc Biol. 2013; 33: e39-46.
- Dronjak S, Gavrilovi? L, Filipovi? D, Radojci? MB. Immobilization and cold stress affect sympatho-adrenomedullary system and pituitary- adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol Behav. 2004; 81: 409-415.
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016; 6: 603-621.
- Leppäluoto J, Westerlund T, Huttunen P, Oksa J, Smolander J, Dugué B, et al. Effects of long-term whole-body cold exposures on plasma concentrations of ACTH, beta-endorphin, cortisol, catecholamines and cytokines in healthy females. Scand J Clin Lab Invest. 2008; 68: 145-153.
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991; 12: 118-134.
- Beppe GJ, Mamadou Astadjam M, Fedaski Christ-Roi F. Evaluation of the neuroprotective effects of hydroethanolic extract of diospyros mespilliformis trunk bark (ebenacea) on diazepam-induced amnesia in mice. J Exp Molec Biol. 2023; 24: 29-40.
- Fontella FU, Siqueira IR, Vasconcellos AP, Tabajara AS, Netto CA, Dalmaz C. Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res. 2005; 30: 105-111.
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996; 93: 3908-3913.
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998; 1: 69-73.
- Brien SE, Smallegange C, Gofton WT, Heaton JP, Adams MA. Development of a rat model of sexual performance anxiety: effect of behavioural and pharmacological hyperadrenergic stimulation on APO-induced erections. Int J Impot Res. 2002; 14: 107-115.
- Bal E, Murat N, Demir O, Soner BC, Can E, Gidener S, et al. Restraint stress impairs erectile responses in rats. Tohoku J Exp Med. 2009; 217: 239-242.
- Maeda K, Tsukamura H. The impact of stress on reproduction: are glucocorticoids inhibitory or protective to gonadotropin secretion? Endocrinology. 2006; 147: 1085-1086.
- de Souza DB, Silva D, Cortez CM, Costa WS, Sampaio FJ. Effects of chronic stress on penile corpus cavernosum of rats. J Androl. 2012; 33: 735-739.
- Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. 2000; 57: 1012-1030.
- Hauger RL, Saelzler UG, Pagadala MS, Panizzon MS. The role of testosterone, the androgen receptor, and hypothalamic-pituitary- gonadal axis in depression in ageing Men. Rev Endocr Metab Disord. 2022; 23: 1259-1273.
- Whirledge S, Cidlowski JA. A role for glucocorticoids in stress- impaired reproduction: Beyond the hypothalamus and pituitary. Endocrinology. 2013; 154: 4450-4468.
- Wagenmaker ER, Moenter SM. Exposure to acute psychosocial stress disrupts the luteinizing hormone surge independent of estrous cycle alterations in female mice. Endocrinology. 2017; 158: 2593-2602.
- de Oliveira RM, Aparecida Del Bel E, Mamede-Rosa ML, Padovan CM, Deakin JF, Guimarães FS. Expression of neuronal nitric oxide synthase mRNA in stress-related brain areas after restraint in rats. Neurosci Lett. 2000; 289: 123-126.
- López-Calderón A, Ariznavarreta C, González-Quijano MI, Tresguerres JA, Calderón MD. Stress induced changes in testis function. J Steroid Biochem Mol Biol. 1991; 40: 473-479.
- Tohei A, Tomabechi T, Mamada M, Akai M, Watanabe G, Taya K. Effects of repeated ether stress on the hypothalamic-pituitary-testes axis in adult rats with special reference to inhibin secretion. J Vet Med Sci. 1997; 59: 329-334.
- Juárez-Rojas L, Vigueras-Villaseñor RM, Casillas F, Retana-Márquez S. Gradual decrease in spermatogenesis caused by chronic stress. Acta Histochem. 2017; 119: 284-291.
- Tian P, Lv P, Shi W, Zhu M, Cong B, Wen B. Chronic stress reduces spermatogenic cell proliferation in rat testis. Int J Clin Exp Pathol. 2019; 12: 1921-1931.
- Zafir A, Banu N. Induction of oxidative stress by restraint stress andcorticosterone treatments in rats. Indian J Biochem Biophys. 2009; 46: 53-58.
- Xiong X, Zhang L, Fan M, Han L, Wu Q, Liu S, et al. β-Endorphin induction by psychological stress promotes leydig cell apoptosis through p38 mapk pathway in male rats. Cells. 2019; 8: 1265.
- Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed. 2016; 14: 231-240.
- Ben Ali H, Atig F, Mehri S. Analyse du statut oxydatif spermatique chez des patients infertiles. Basic Clin Androl. 2012; 22: 233-240.
- Benedetti S, Tagliamonte MC, Catalani S, Primiterra M, Canestrari F, De Stefani S, et al. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online. 2012; 25: 300-306.
- Ayad B, Omolaoye TS, Louw N, Ramsunder Y, Skosana BT, Oyeipo PI, et al. Oxidative Stress and Male Infertility: Evidence From a Research Perspective. Front Reprod Health. 2022; 22: 822257
- Stephens MA, Wand G. Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol Res. 2012; 34: 468-483.
- Hu GX, Lian QQ, Lin H, Latif SA, Morris DJ, Hardy MP, et al. Rapid mechanisms of glucocorticoid signaling in the Leydig cell. Steroids. 2008; 73: 1018-1024.
- Nateghian Z, Aliabadi A, Aliabadi E. Effects of stress-induced glucocorticoids on reproductive dysfunction in men stress-induced glucocorticoids and male infertility. 2021; 11: 3780-3790.