Carbimazole-Induced Hepatotoxicity - Avoid Rechallenging
- 1. Waikato Clinical Campus, University of Auckland, New Zealand
ABSTRACT
All thioamide anti - thyroid medications are known to be associated with liver dysfunction. Cholestasis is most commonly reported in association with carbimazole/ methimazole. We report a patient with Graves’ disease, who developed acute Hepatocellular dysfunction associated with carbimazole. There is limited data available on re -exposure in this situation. The patient rechallenged herself twice and subsequent exposures resulted in an increasingly shorter time to symptom onset until finally even a single 5mg tablet rapidly provoked severe symptoms and abnormal liver function tests.
This case supports the need to avoid anti - thyroid medication rechallenge in patients who develop significant liver dysfunction following their use and identifies that carbimazole may be associated with acute Hepatocellular dysfunction and not just Cholestasis.
KEYWORDS
• Antithyroid agents
• Drug-induced liver injury
• Thyrotoxicosis
• Graves’s disease
CITATION
Karalus M, Jade Tamatea AU, Conaglen JV, Elston MS (2016) Carbimazole-Induced Hepatotoxicity - Avoid Rechallenging. JSM Thyroid Disord Manag1(1): 1001.
ABBREVIATIONS
ATD: Anti - Thyroid Drugs; PTU: Propylthiouracil; CBZ: Carbimazole; MMZ: Methimazole; ALT: Alanine Transaminase
INTRODUCTION
Initial therapy for thyrotoxicosis is commonly with anti - thyroid drugs (ATD). Serious side effects from ATD such as agranulocytosis and hepatic dysfunction, although rare but can be life - threatening [1]. Propylthiouracil (PTU) is now well known to be associated with hepatotoxicity, particularly in the paediatric population, increasing liver failure in the exposed population by a factor of 17 [2]. Carbimazole (CBZ) is a 3 - carbethoxy methimazole (MMZ) derivative, rapidly metabolized to MMZ [3]. As compared to the hepatocellular dysfunction resulting from PTU, CBZ/MMZ hepatotoxicity is usually cholestatic [4]. Patients whose liver function tests deteriorate whilst on ATD are usually advised against further use of these medications, although there are limited data on the actual risk of rechallenge in this situation.
We present a patient with Graves’ disease, who developed acute hepatitis associated with CBZ and, despite medical advice to the contrary, rechallenged herself not once, but twice.
CASE PRESENTATION
A 54 year - old woman presented with a 2 month history consistent with thyrotoxicosis. Clinically she was mildly thyrotoxic with a diffuse goitre, a thyroid bruit and moderate - severe thyroid eye disease. Biochemistry demonstrated mild thyrotoxicosis with normal liver function tests (LFTs) (Figure 1 and Table 1).
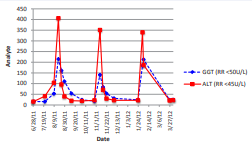
Figure 1 Temporal pattern of liver function test results following carbimazole use. GGT = gamma-glutamyltransferase; ALT = alanine aminotransferase
Table 1: Peak recorded liver function tests.
| Baseline | Initial CBZ | |||
| Bilirubin (RR < 24umol/L) | 5 | 13 | 16 | 14 |
| ALP (RR 40 -120U/L) | 58 | 199 | 130 | 146 |
| GGT (RR < 50U/L) | 24 | 215 | 141 | 184 |
| ALT (RR < 45U/L) | 22 | 406 | 351 | 340 |
| AST (RR < 35U/L) | - | 121 | 92 | 44 |
| Abbreviations: CBZ = Carbimazole; RR = Reference Range; ALP = Alkaline Phosphatase; GGT = Gamma-Glutamyltransferase; ALT = Alanine Aminotransferase; AST = Aspartate Aminotransferase | ||||
Initial therapy with 20mg CBZ once daily was reduced after 2 weeks to 10mg per day. One month after starting CBZ, routine liver function testing revealed abnormal LFTs particularly alanine transaminase (ALT) (Figure 1 and Table 1). CBZ was stopped. There was no evidence of viral or autoimmune hepatitis and no other potential hepatotoxic agents were identified. Ten days after stopping CBZ, her LFTs returned to normal. The patient was advised that all ATD should be avoided and due to her severe thyroid eye disease a thyroidectomy was recommended, with the possibility of radioiodine with glucocorticoid cover as an alternative. The patient declined definitive treatment and remained off all therapy.
Within two months, she again became overtly thyrotoxic. Soon afterwards, routine monitoring blood tests demonstrated abnormal liver results (Figure 1 and Table 1) and on phoning the patient she reported having recently restarted low dose CBZ (<10mg per day, exact duration unclear but < two weeks). She was again strongly advised against ever taking ATD. Her LFTs normalised within one month.
Markedly abnormal LFTs were identified again two months later (Figure 1 and Table 1). On questioning, the patient reported having rechallenged herself with a single 5mg tablet of CBZ three days earlier. Approximately 20 minutes after taking the dose, she started vomiting. Her LFTs returned to normal and the patient eventually elected to undergo a total thyroidectomy, which was uncomplicated. No further episodes of acute liver dysfunction were noted over the following three years.
DISCUSSION
All anti - thyroid medications (CBZ, MMZ and PTU) are known to be associated with liver dysfunction [1]. This rare side effect has an overall incidence of less than 0.5% [5], and for CBZ/ MMZ the risk appears to correlate with dose [6]. Carbimazole hepatotoxicity occurs more frequently in populations over the age of 40 [5] in contrast to Propylthiouracil (PTU) in which it is more common in children [2]. The exact mechanism of CBZ/MMZ - induced hepatotoxicity remains unclear with hypersensitivity, cell mediated immunity and drug reactions being hypothesised [5]. Cholestatic injury is the most common type of hepatic dysfunction reported in association with CBZ/MMZ [4]. Less commonly, hepatic injury due to necro - inflammatory, granulomatous hepatitis and steatosis have been reported associated with CBZ/MMZ [4]. The reported length of time for CBZ - induced hepatitis to become manifest is variable, ranging from a few days to five months [5]. Whilst PTU is now well established to result in potentially fulminant hepatotoxicity [2] CBZ/MMZ is usually reversible although deaths have been reported [7,8].
Carefully documented cases showing recurrent hepatitis after CBZ/MMZ rechallenge are rare. A MEDLINE search using the search terms ‘carbimazole or methimazole or thioamides or anti thyroid agents’ and ‘hepatotoxicity or liver injury’ was performed. Ten case reports were identified reporting recurrent hepatotoxicity after at least one CBZ/MMZ rechallenge [9-19]. Common across these reports were: symptoms of pruritus, jaundice, darkened urine, abdominal discomfort and vomiting; a clear chronological relationship between CBZ/MMZ intake and onset of symptoms; and all episodes being reversible. Also apparent from the literature, and similar to our patient, was that the re - introduction of CBZ resulted in a shorter interval for the patients to develop symptoms. This suggests possible increased sensitivity following re - exposure to CBZ. In only one of these reports [11] the hepatotoxicity appeared to be predominantly Hepatocellular with the remainder Cholestatic.
The current case has several limitations. Due to the unexpected CBZ rechallenges, patient monitoring and blood tests were not conducted in a controlled setting and therefore peak results are unknown and unfortunately the first rechallenge dose and duration were not recorded. A liver biopsy was also not performed which would have provided histological confirmation of hepatitis and the subtype. However, given the absence of other potential causative agents this case clearly demonstrated hepatotoxicity temporally associated with CBZ use, with subsequent exposures resulting in a shorter time to symptom onset.
This case strongly supports the need to avoid ATD rechallenge in patients who develop significant liver dysfunction following ATD use and identifies that CBZ may be associated with acute Hepatocellular dysfunction and not just Cholestasis.








































































































































