Repellent Effect of Borneols and Bornanediols against Aedes Albopictus: in Search of New Natural Repellents
- 1. Laboratoire International Associe (LIA), Faculté des Sciences, Université d’Antananarivo, Madagascar
- 2. Département d’Entomologie Médicale, Faculté des sciences, Université d’Antananarivo, Madagascar
- 3. Institut de Chimie et de Biochimie Moléculaires et Supramoléculaires (ICBMS), Université de Claude Bernard Lyon 1/Centre National de Recherche Scientifique (CNRS), France
Abstract
Aedes mosquitoes are the main vectors of several arboviruses. The use of repellents is one of the most effective strategies to prevent mosquito bites and limit host-vector interactions. This work describes the synthesis of borneols and isoborneols from camphor as well as 2-exo-3-exo-bornanediols via the camphorquinone and the evaluation of their repellents properties. The repellent activity of the synthesized products was evaluated using a tunnel olfactometer. 2R-(−)-isoborneol (1a), 2S-(+)-isoborneol (1b), (+)-1R-2-exo-3-exo-bornanediol (3a) and (+)-1S-2-exo-3-exo-bornanediol (3b) demonstrated repellent activity against Aedes albopictus at different doses ranging from 1 to 40 mg. In addition, 2S-(+)-borneol (2a) and 2R-(+)-borneol (2b) exhibited repellency at doses of 0.25 to 10 mg. Human volunteer tests indicated that (+)-1R-2-exo-3-exo-bornanediol (3a) and (+)-1S-2-exo-3-exo-bornanediol (3b) provided an average of 50% protection against Aedes albopictus during the 60-minute test. The repellent effect of 2S-(+)-isoborneol (1b) was not satisfactory, and no synergistic effect was observed with the mixture of the two bornanediols (3a and 3b). We conclude that terpene diols act as the pharmacophore responsible for the repellent activity.
KEYWORDS
- Bornanediols
- Borneols
- Isoborneols
- Repellent activity
- Aedes albopictus
CITATION
Ramarosandratana NH, Borrego LG, Duclos MC, Andrianjafy MT, Métay E, et al. (2025) Repellent Effect of Borneols and Bornanediols against Aedes Albopictus: in Search of New Natural Repellents, Tanzania. JSM Trop Med Res 6(1): 1022.
ABBREVIATIONS
DEET: Di-methyl m toluamide; PMD: para-menthane- 3,8-diol; NMR: Nuclear Magnetic Resonance; mp: Melting Point; VRA: Vaporisation Rate Analysis; EtOH: ethanol; r.t: room temperature; EtOAc: Ethyl acetate; ESI: Electrospray Ionization; TLC: Thin Layer Chromatography;
INTRODUCTION
Aedes mosquitoes are the primary vectors of several arboviruses such as Dengue, Zika, and Chikungunya [1-3]. The use of repellents is one of the most effective strategies for avoiding mosquito bites to limit host-vector interactions and subsequently reduce the transmission of associated diseases [4-6]. Throughout history, humans have used various methods to protect themselves from mosquito bites, ranging from the use of smoke [7,8], to the cultivation of plants with repellent properties near human settlements [9]. Topical application of essential oils as repellents is a common practice [10]. Monoterpenoid molecules with acyclic, monocyclic, bicyclic structures and with mono-alcohol, diol, ketone and aldehyde functions are the main constituents of plant essential oils claimed to be repellent. Examples include terpinen-4-ol, terpinene, 1,8-cineole, geraniol, linalool and myrcene, which are present in oils from Petroselinum crispum, Citrus aurantifolia L., Eucalyptus citriodora, Lavandula angustifolia, Cinnamomum camphora, Zingiber officinale, Mentha L. spp. However, these natural repellents provide protection for a limited duration and require frequent re- applications to maintain effectiveness [11]. In response to consumer demand, highly effective synthetic repellents have been developed. For instance, dimethylphthalate and dibutylphthalate were discovered in 1929, Indalone ® in 1937 and ethyl hexanediol in 1939. Today, the most widely used and effective repellent is DEET, discovered in 1953. Other products such as IR 3535 (introduced in 1970) and picaridin (in 1988) have been used successfully in formulations against mosquitoes and other hematophagous insects [12]. Unfortunately several synthetic repellents have been associated with human health effects, such as childhood encephalopathy, urticaria, blood pressure imbalances, allergic reactions, skin problems and neurotoxic effects [13]. In response to these concerns, there is a growing demand for natural repellents with lower toxicity and ecological impact. Currently, only para-menthane-3,8-diol (PMD), a constituent of Eucalyptus citriodora essential oils is recognized as an effective natural repellent although it is often obtained by hemisynthesis from citronellal [14-16]. Various terpenes and compounds containing alcohol, ketone or ester groups have shown promising repellent properties [17-19]. However, the duration of efficacy remains a major challenge due to the high vapor pressure of these natural family compounds. In our previous study, the four stereoisomers of PMD exhibited different degrees of protection against the Asian tiger mosquito (Aedes albopictus), with 1R-cis-PMD being the most potent repellent [16]. Borneol and its isomers are found at low levels in several essential oils with repellent activity. This molecule has been detected in Rosmarinus officinalis L. at levels ranging from 0.1% to 6.4% [20], in Amomum biflorum extract at 0.1% [21] and in Artemisia argyi H, at 1.8 to 6.2%, with isoborneol at 1.4 to 2.6% [22]. Isoborneol and borneol are thought to have repellent activity against blood-sucking insects. However like many other natural compounds, their effectiveness decays rapidly [11]. Certain natural compounds like PMD show a strong repellent activity against mosquitoes such as Ochlerotatus taeniorhynchus [23]. New effective and inexpensive strategies are still needed to protect humans from mosquito bites and studying the efficacy of mono- or di-hydroxylated derivatives of bornane (or camphane) seems particularly interesting because of their ubiquity in plants.
In this work, we aim to establish the repellent properties of borneol, isoborneol and bornanediol stereoisomers against Aedes albopictus. With PMD as a reference, we hypothesize that diols, in general, can be considered pharmacophores for repellent activity in the terpene series, with lower vapor pressure and offering longer- lasting protection compared to the corresponding mono- alcohols. Chemical modifications of these bio-sourced and safe molecules may further enhance their duration of effectiveness as repellents. This research involves the selective chemical synthesis of borneol and its derivatives, followed by bioassays to assess their repellent properties against Aedes albopictus.
MATERIALS AND METHODS
Chemical analysis
Nuclear Magnetic Resonance: NMR (1H-NMR and 13C-NMR) analysis was performed for structural identification of the molecules.
Optical rotation: Optical rotation of chiral molecules was determined using a polarimeter (Bellingham Stanley ADP 220).
Melting point: Melting point analysis of the products was determined using a SMP3 melting point meter.
Vaporization rate analysis (VRA): To determine the thermal properties of the synthesized compounds, 100 mg of each compounds of 2S-(+)-isoborneol (1b), 2S- (+)-borneol (2a), 2R-(−)-borneol (2b), (−)-2R-2-exo-3- exo-bornanediol (3a) and 1R-cis-PMD were placed in a 30 mL beaker at 50°C for 120 min. The weight of each sample was checked every 15 min.
Synthesis of the products
In plant extracts, the proportion of borneol is generally small [20,22,24,25]. To obtain sufficient quantities for biological tests, synthesis or hemisynthesis is required. Conversely, both isomers of camphor are commercially available at relatively low prices, making them ideal as starting materials. Furthermore, to our knowledge, although many essential oils containing hydroxylated camphane have been described as repellent in literature [26-29], the insect activities of borneol and bornanediol isomers have never been tested under controlled conditions, either in tunnel olfactometers or on volunteers.
Synthesis of borneol (2) and isoborneol (1)
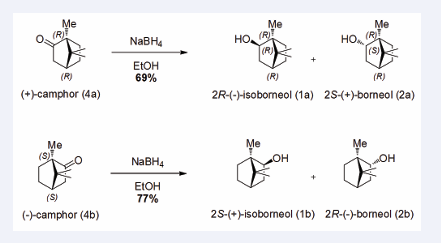
From the two enantiomers of camphor (4), two isomers of borneol (2) and two isomers of isoborneol (1) can be prepared. The reduction of (+)-camphor (4a) and (−)-camphor (4b) with NaBH4 leads to the corresponding alcohols 2S-(+)-borneol (2a), 2R-(−)-borneol (2b) and both (+) and (−)-isoborneol (1a and 1b) (Figure 1),
Figure 1: Reduction of enantiopure camphor (4) enantiomers into different enantiomers of borneol (2) and isoborneol (1).
with good yields. The isoborneol/borneol ratio is 82/18. Due to the steric hindrance of camphor (4), the hydride attacks on the opposite side of the 1,4 bridge, placing the hydroxyl group on the same side as the bridge.
Reduction of camphor (4): NaBH4 (100 mol%) was added in portions to a 0.6 M solution of camphor (4) (100 mol%) in EtOH at room temperature (r.t). The reaction was monitored by TLC. Once the starting product was fully consumed, the reaction mixture was quenched with a saturated aqueous solution of NH4Cl. The aqueous phase was extracted with CH2Cl2 (3×40 mL) and the organic phase was dried by using anhydrous MgSO4. The solvent was then evaporated under reduced pressure. The crude product was purified by flash chromatography (CombiFlash next Gen 300+) using a gradient of Cyclohexane/EtOAc (4:1 to 1:1) for 20 minutes, followed by 100% of EtOAc for an additional 20 minutes, at a flow rate of 30 mL/min.
Synthesis of 2R-(−)-isoborneol (1a) and 2S-(+)-borneol (2a) They were prepared according to the general procedure vide supra from 1 g of (−)-camphor (4a) (6.56 mmol), 248 mg of NaBH4 (6.56 mmol) and 10 mL of EtOH. After 18 hours of reaction, 704.1 mg of product (4.57 mmol, 69% yield) were obtained as a mixture of 2R-(−)-isoborneol (1a) and 2S-(+)-borneol (2a), which were separated by flash chromatography.
2R-(−)-isoborneol (1a): 570 mg (3.70 mmol, 56% yield) of (1a) was obtained as a white solid; mp 204 °C; [α] D20 −33.3 (c 1, CHCl3) (mp 212 to 214 °C; [α] 20 −34 [30,31] ); 1H-NMR (500 MHz, CDCl3): δ 3.57 (dd, J = 3.9 and 7.6 Hz, 11.5 Hz, 1H), 0.98 (s, 3H), 0.99-0.89 (m, 2H), 0.86 (s, 3H),0.78 (s, 3H) ppm; 13C-NMR (125 MHz, CDCl3): δ 79.8, 49.0,46.4, 45.1, 40.4, 34.0, 27.3, 20.6, 20.2, 11.4 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C HNaO177.1250; found 177.1250 (-0.3ppm).
2S-(+)-borneol (2a): 134.1 mg (0.87 mmol, 13% yield) of (2a) in the form of white solid; m p: 202 °C; [α]D20 +37.8 (c 1, CHCl3) (mp 202 °C; [α] 20 +37 [30,31]); 1H-NMR (500 MHz, CDCl3): δ 4.00 (ddd, J = 1.8, 3.5 and 10.0 Hz, 1H), 2.30-2.23 (m, 1H), 1.91-1.85 (m, 1H), 1.76-1.69 (m, 1H), 1.62-1.61 (m, 2H), 1.27-1.25 (m, 2H), 0.94 (dd, J = 3.5 and 13.4 Hz, 1H), 0.86 (s, 3H), 0.85 (s, 3H), 0.84 (s, 3H) ppm;13C-NMR (125 MHz, CDCl3): δ 77.5, 49.6, 48.2, 45.2, 39.1,28.4, 26.0, 20.3, 18.8, 13.5 ppm. HRMS (ESI) m/z: [M + Na]+Calcd for C10H18NaO2 177.1250; found 177.1247 (1.7 ppm).
Synthesis of 2S-(+)-isoborneol (1b) and 2R-(−)- borneol (2b)
They were prepared according to the general procedure vide supra from 3 g of (−)-camphor (4b) (19.7 mmol), 710 mg of NaBH4 (19.7 mmol) and 30 mL of EtOH. After 24 hours of reaction, 2.34 g of product were obtained (15.2 mmol, 77% yield) in the form of a mixture of 2S-(+)- isoborneol (1b) and 2R-(−)-borneol (2b), which were separated by flash chromatography.
2S-(+)-isoborneol (1b): 1.79 g (11.6 mmol, 59% yield) of (1b) in the form of white solid was obtained; mp 204°C; [α]D 20 +35.5 (c 1, CHCl3 ) (mp 212 to 214 °C; [α]D 20 +37 [30,31]) ; 1 H-NMR (500 MHz) and 13C-NMR (125 MHz): spectroscopy RMN identic on 2R-(−)-isoborneol (1a). HRMS (ESI) m/z: [M + Na]+ Calcd for C10H18NaO2 177.1250; found 177.1246 (2.3 ppm).
2R-(−)-borneol (2b): 547.0 mg (3.5 mmol, 18% rdt) of (2b) in the form of white solid was obtained; mp 202°C; [α]D 20 −37.8 (c 1, CHCl3 ) (mp 204 to 208°C; [α]D 20 −37 [30,31]); 1 H-NMR (500 MHz) and 13C-NMR (125 MHz): NMR spectroscopy data are similar to 2S-(+)-borneol (2a). HRMS (ESI) m/z: [M + Na]+ Calcd for C10H18NaO2 177.1250; found 177.1247 (1.6 ppm).
Synthesis of bornanediol (3)
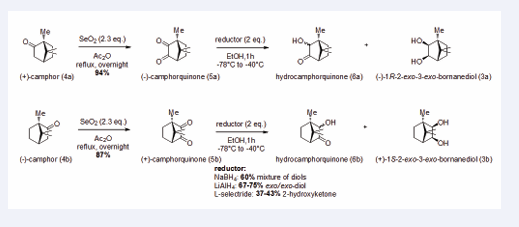
Bornanediols were obtained from camphor quinones (5) by reduction with lithium aluminum hydride.
Camphor (4) can be transformed into the diketone
camphor quinone (5) with excellent yields via a Riley reaction using selenium dioxide and acetic anhydride (Figure 2).
Figure 2: Synthesis of bornanediol and hydrocamphorquinone (6) from enantiopure camphor (4)
Figure 3: Products synthesized
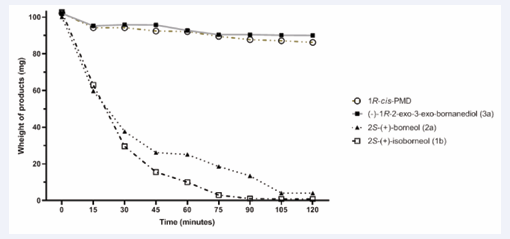
Figure 4: Vaporization rate analysis of the compounds.
This diketone can then be reduced to the corresponding diols, as analogues of PMD. To investigate the stereoselectivity of this reduction, the reactions were carried out at -78°C using NaBH4, LiAlH4 and L-selectride. The reaction with NaBH4 gived a mixture of four possible diols. L-selectride partially reduced the diketone to the corresponding hydroxyketones. Surprisingly, only the diol exo/exo was obtained when using LiAlH4. The hydride addition occurs primarily on the opposite face of the bridge due to steric hindrance, yielding the exo isomers as major products. These products have never been tested as mosquito repellents and they are of interest, as they are dioxygenated terpenoids like PMD, the only natural product with proven efficacy.
(−)-1R-2-exo-3-exo-bornanediol (3a)
A 1M solution of LiAlH4 in THF (12 mL, 12 mmol) was added dropwise to a solution of (−)-camphorquinone (5a) (1 g, 6 mmol) in anhydrous THF (30 mL) at −78°C and under argon atmosphere. Once the starting product is fully consumed (2 hours), the reaction medium was quenched with a saturated aqueous solution of NH4Cl. The aqueous phase was extracted with EtOAc (3 x 40 mL) and the organic phase was dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure. The crude product was purified by flash chromatography (CombiFlash next Gen 300+) using a gradient of cyclohexane/EtOAc (4:1 to 1:1) for 20 minutes, followed by 100% EtOAc for an additional 20 minutes, at a flow rate of 60 mL/min.
(−)-1R-2-exo-3-exo-bornanediol (3a): 769.1 mg (4.51 mmol, 75%) of a white solid was obtained, mp 251 °C; [α] 20 -14.7 (c 0.8, CHCl ) ((mp 262 to 263 °C; [α] 20 -19.3 [32]).
1H-NMR (500 MHz, CDCl3): δ 3.78 (d, J = 7.0 Hz, 1H), 3.54 (d, J = 7.0 Hz, 1H), 1.67-1.64 (m, 2H), 1.48-1.44 (m, 1H), 1.08 (s, 3H), 0.99-0.96 (m, 2H), 0.91 (s, 3H), 0.81 (s, 3H) ppm; 13C-NMR (125 MHz, CDCl3): δ 80.6, 76.8, 52.7, 49.6, 47.3, 34.3, 25.0, 22.4, 21.6, 11.6 ppm. HRMS (ESI) m/z: [M + Na]+ Calcd for C10H18NaO2 193.1199; found 193.1196 (1.5 ppm).
(+)-1S-2-exo-3-exo-bornanediol (3b) was prepared by the same procedure as 3a, using (+)-camphorquinone (5b) as the starting material.
(+)-1S-2-exo-3-exo-bornanediol (3b): 683 mg (4.01 mmol, 67%) of a white solid was obtained. mp 251°C; [α] 20 +11.9 (c 0.8, CHCl ); 1H-NMR (500 MHz) and 13C-NMR (125 MHz): spectroscopic data are similar to those of (3b). HRMS (ESI) m/z: [M + Na] + Calcd for C H NaO 193.1199;found 193.1194 (2.5 ppm).
Mosquitoes
Aedes albopictus was used in all the experiments. Larvae, pupae and adults were collected at Tsimbazaza Zoological Park in the bamboo habitat (S18°55’42.31”; E47°31’38.23”). The collected specimens were transported and raised in the insectarium of the International Associated Laboratory, located at the IST Ampasapito campus under the following conditions: temperature of 26±4°C, relative humidity of 65±5% and a photoperiod of 12/12 hours. In breeding cages, the adults were fed with cotton soaked in a 10% sucrose solution. Guinea pig blood was used as the food source for the females. The larvae were placed in rearing tanks and fed with powdered dog biscuits. The raising generations were used for the biological tests.
Bioassay
To evaluate the repellent properties of the products, olfactometer tests were carried out in a tunnel [16,33]. 15 females Aedes albopictus mosquitoes, aged between 5 and 12 days and fasted for 12 hours, were released into the olfactometer. Test and control filter papers were placed at the ends of the device (treated and control zones). To allow the mosquitoes to adapt to their new environment, they were left in the release zone, in the middle of the tunnel surrounded by two polystyrene barriers, for 20 minutes before the start of the test. At the beginning, the barriers were opened to allow the mosquitoes to move freely around the tunnel. Results were collected every five minutes for twenty minutes, counting mosquitoes in the neutral, control and treated compartments. Four replicates were performed for each product dose. Blank tests were performed regularly.
The mosquito activity index was calculated using the following formula:
IA(%)= T+P x100 N
With:
AI (%) = activity index
T= number of mosquitoes in control compartment P= number of mosquitoes in treated compartment N= total number of mosquitoes to be tested
The repulsion index for each product was calculated using the following formula:
IR(%)= T - P x100 T
With: RI (%) = Repulsion Index
T= number of mosquitoes in control compartment P= number of mosquitoes in treated compartment
5.8. Tests on volunteers
Testing in the olfactometer tunnel is not sufficient to determine product activity. In the case of direct testing on volunteers, the product emitted from the skin of the volunteers acts as an attractant for the mosquitoes. In fact, one of the effects of the repellent is to mask the attractive properties of the kairomone emitted by the host [34]. Moreover, there is no air renewal in the tunnels which had a volume of 5580 cm3. The most efficient isomers were tested directly on volunteers to observe the real repellent activity of the products after tunnel olfactory test. The experiment was conducted according to the WHO protocol [35], as described below. Fifty nulliparous female mosquitoes, aged between 5 and 12 days, previously fasted for 12 hours were placed in a cage (40 x 40 x 40 cm3) using a mouth aspirator. The forearms of the volunteers were cleaned with water and then with 1.5 mL of 90 % ethanol. The area of the forearm used for the test is approximately 600 cm2. A latex glove was used to cover the hand. The test began by introducing the volunteer’s forearm (control or untreated) into the test cage during three minutes. Mosquitoes that landed and attempted to bite were counted and then the forearm was shaken to remove them [35,36]. This operation was repeated every thirty minutes until 180 minutes had elapsed. Four volunteers including 2 women and 2 men, aged 20 to 30 years were used for each test and for each product in order to avoid bias due to the influence of sex and age on mosquito response [36]. Volunteers were also asked not to use perfumed or repellent products before and during the tests to avoid biasing the results. Smokers were not selected for the experiment.
DATA ANALYSIS
Data analysis and graphs were performed using GraphPad Prism software version 8.4.2. The effect of each compound at each dose using the tunnel test was compared by t-test, with a significance level of 0.05 to reject the null hypothesis.
RESULTS
Synthesized and tested products
Six products were synthesized including the four stereoisomers of borneol: 2R-(−)-isoborneol (1a), 2S-(+)- isoborneol (1b), , 2S-(+)-borneol (2a), 2R-(−)-borneol (2b) and two isomers of bornanediol: (−)-1R-2-exo-3- exo-bornanediol (3a) and (+)-1S-2-exo-3-exo-bornanediol (3b).
The results of vaporization rate analysis (VRA) are shown in fig.4. A difference in volatility between the different compounds and (1R)-(+)-cis-PMD was observed. 2S-(+)-borneol (2a) and 2S-(+)-isoborneol (1b) exhibited a higher volatility compared to (−)-bornanediols (3a) and (1R)-(+)-cis-PMD which showed similar vaporization rate. Borneol and isoborneol completely evaporated after 120 min at 50°C of the experiment.
Bioassays results
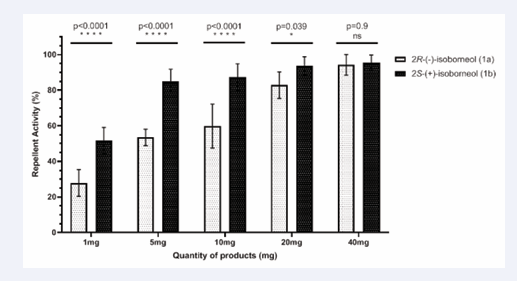
Tunnel tests with (+) and (−)-isoborneol (1b and 1a): For both (+) and (−)-isoborneol (1b and 1a), the following doses were tested: 1 mg, 5 mg, 10 mg, 20 mg and 40 mg. Repellent activities against Aedes albopictus were observed for all quantities with Repellency Index (RI) values ranging from 25 to 90%. The repellent effect of 2R-(−)-isoborneol (1a) varied with the amount tested (Figure 5), showing a significant dose-response relationship. As the amount of 1a increased, so did its repellent effect. At 1 mg, the repellent effect was low (RI=27%) while at 40 mg a much higher effect was observed (RI=94%). On the other hand, 2S-(+)-isoborneol (1b) exhibited repellent activity RI=51% at 1 mg. Increasing the dose led to a plateau effect with repellent activities remaining between 85% and 94% from 5 mg to 40 mg (Figure 5).
Figure 5: Repellent effects of (+) and (−)-isoborneol (1b and 1a) by tunnel olfactometer.
Overall, 2S-(+)-isoborneol (1b) demonstrated significantly greater repellent activity than 2R-(−)- isoborneol (1a) particularly at lower doses (1 mg, 5 mg and 10 mg).
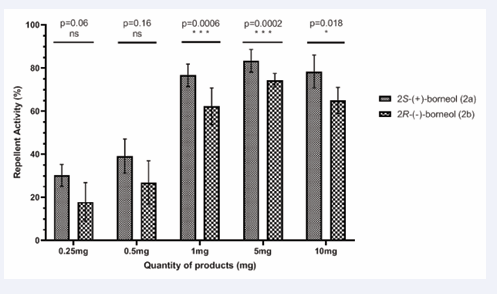
Tunnel tests with (+) and (−)-borneol (2a and 2b): Five doses of both (+) and (−)–borneol (2a, 2b) were tested using the tunnel device: 0.25 mg, 0.5 mg, 1 mg, 5 mg and 10 mg. Both compounds showed repellent activity at all doses tested with effects varying according to the quantity used (Figure 6).
Figure 6: Repellent effects of (+) and (−)–borneol (2a, 2b) at doses- of 0.25 mg, 0.5 mg, 1 mg, 5 mg, and 10 mg for tunnel tests
For 2R-(−)-borneol (2b), the repellent effect was 17% at 0.25 mg. As the dose increased, so did the repellency with RI values of 27%, 62% and 74% for 0.5 mg, 1 mg and 5 mg, respectively. In contrast, at the highest dose of 10 mg, the repellent activity slightly decreased to 65%.
2S-(+)-borneol (2a) consistently exhibited higher repellent activity than 2b. Statistically, significant differences between the two enantiomers were observed at doses of 1 mg, 5 mg and 10 mg. However, in all cases, both enantiomers demonstrated comparable repellent activity against Aedes albopictus.
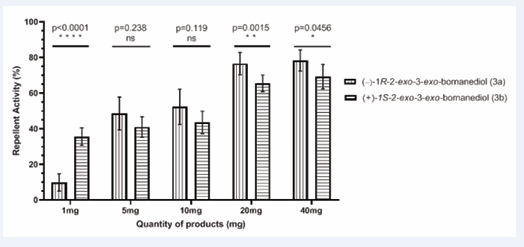
Tunnel evaluation of (−)-2R -2-exo-3-exo- bornanediol (3a) and (+)-1S -2-exo-3-exo-bornanediol (3b): Both enantiomers of bornanediol (3a and 3b) exhibited repellent activity against Aedes albopictus at all tested doses (Figure 7).
Figure 7: Repellent effects of (+)-1S- and (−)-2R-2-exo-3-exo-bornanediol (3b and 3a) at doses of 1 mg, 5 mg, 10 mg 20 mg and 40 mg for tunnel tests
As in previous results, the repellent effects were also dose-dependent. For (−)-2R -2-exo-3-exo-bornanediol (3a), the repellent effect at 1 mg was lower (RI = 10%). However, it increased to 48% at 5 mg and remained unchanged at 10 mg. At the higher doses of 20 and 40 mg, the repellency stabilized at around 76%.
For (+)-1S-2-exo-3-exo-bornanediol (3b), doses of 1 mg, 5 mg and 10 mg produced similar repellent effects with an average RI of 43%. A significant increase in repellency was observed at the 20 mg dose with an RI of 66% which remained stable at 40 mg.
In comparison, both bornanediols showed similar repellent activity at all levels tested, except at 1 mg, where the repellency of (3b) is significantly higher than that of (3a).
Human volunteer tests
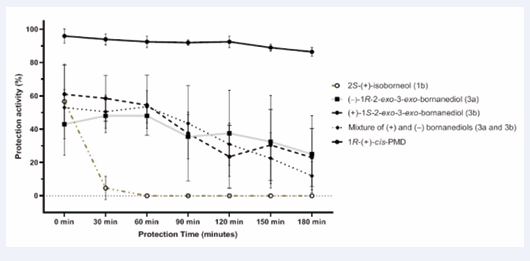
As 2S-(+)-isoborneol (1b) exhibited strong repellent activity at 5 mg in the tunnel olfactometer and both bornanediols (3a and 3b) have diol functional groups similar to PMD, they were selected for testing on volunteers ’forearms at a dose of 200 mg. The results are shown in Figure 8.
Figure 8: Comparison of protective activity on volunteers between 2S-(+)-isoborneol (1b), (+)-1S-2-exo-3-exo-bornanediol (3b) and (−)-2R-2- exo-3-exo-bornanediol (3a) to PMD, all tested at a dose of 200 mg
2S-(+)-isoborneol (1b) provided a protection rate of 56%. This protection rapidly declined to 5% after 30 minutes and then disappeared entirely thereafter.
(+)-1S-2-exo-3-exo-bornanediol (3b) showed 60% protection at the beginning of the test. The protection gradually decreased over time but it still offered protection after 60 minutes (56%) and after 90 minutes (38%). The repellent effect remained relatively stable between 20% and 30% from 120 minutes to the end of the test.
(−)-2R-2-exo-3-exo-bornanediol (3a) had an initialeffect of 40 %, which remained stable for the first 60 minutes. After 90 minutes, the protection rate was 35%, slowly decreasing to 25% when the test concluded after 180 min.
Additionally, a mixture of the two bornanediols (3a and 3b) was tested on volunteers to assess a possible synergistic effect as repellents against Aedes albopictus. The mixture demonstrated repellent activity against mosquitoes. At the beginning of the test, it showed a repellent activity of 52% which remained stable for the first 60 minutes. At 90 minutes, the repellent activity decreased to 42% and at the end of the 180-minute test, it dropped to 10%.
Compared to the mixture of the four stereoisomers of PMD, all borneols and bornanediol showed significant lower repellent activity. At the same tested dose (200 mg), 1R-(+)-cis-PMD exhibited over 90% repellent activity for 2 h only decreasing to 85% at the end of the test.
DISCUSSION
In tunnel tests, borneol derivatives: 2S-(+)-isoborneol (1b), 2R-(−)-isoborneol (1a), 2R-(−)-borneol (2b) and 2S-(+)-borneol (2a) exhibit effective repellent activity against Aedes albopictus even at relatively low doses. However, rapid evaporation and diffusion render the product ineffective when applied directly to human volunteers.
Volatility Rate Analysis (VRA) shows that borneol and isoborneol evaporate much faster than their diol counterparts. This rapid evaporation enhances their effectiveness in tunnel olfactometer tests. But on human skin, it leads to minimal protection due to quick dissipation. In contrast, bornanediols (3a and 3b) exhibit lower repellent activities in tunnel tests in comparison with borneols and isoborneols. However, they offer much longer protection for volunteers. VRA indicates these diols have significantly lower evaporation rates than borneols and isoborneols (Figure 4), which helps to explain their superior performance in prolonged protection. In addition, borneol, isoborneol, and bornanediol melt at significantly higher temperatures than PMD isomers, which have melting points between 75 and 79°C [16].
In tests with mixture of bornanediol (3a and 3b), there is an additive effect but no synergistic effect was observed and the stereochemistry did not have a significant impact on repellency efficacy, unlike in the case of PMD [16].
Borneols and isoborneols are highly effective mosquito repellents but their high volatility makes them unsuitable for long-lasting products like creams or sprays. However, they can be used with diffusers as aerial repellents, or planting species such as Lantana camara around homes could serve as a cost-effective and environmentally friendly solution [24,25]. Overall, terpenediols act as a valuable pharmacophore with significant repellent potential, as suspected by other studies [37].
CONCLUSION
During our experiments, we found that the compounds tested, including isoborneols, borneols and bornanediols, all showed repellent activity against Aedes albopictus mosquito. Bornanediols showed significant repellent activity in tests on volunteers. On the other hand, these compounds had low activity compared to PMD.
Although bornanediols are less effective than PMD, they meet the demand for natural repellent products. These diols could be formulated in combination with other repellents for enhanced efficacy. Their inclusion in repellent formulations, although less potent than PMD, could provide natural alternatives for mosquito control.
REFERENCES
- Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. Elife. 2015; 4: 1-18.
- Lwande OW, Obanda V, Lindström A, Ahlm C, Evander M, Näslund J, et al. Globe-Trotting Aedes aegypti and Aedes albopictus: Risk Factors for Arbovirus Pandemics. Vector-Borne Zoonotic Dis. 2020; 20: 71-81.
- Madewell ZJ. Arboviruses and their vectors. South Med J. 2020; 113: 520523.
- Asadollahi A, Khoobdel M, Zahraei-Ramazani A, Azarmi S, Mosawi SH. Effectiveness of plant-based repellents against different Anopheles species: A systematic review. Malar J. 2019; 18: 436.
- Bekele D. Review on insecticidal and repellent activity of plant products for malaria mosquito control. Biomed Res Rev. 2018; 2: 1-7.
- Gillij YG, Gleiser RM, Zygadlo JA. Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour Technol. 2008; 99: 2507-2515.
- Biran A, Cameron C, Ensink J, Smith L. Smoke and Malaria. 2008; 32
- Dube F, Tadesse K, Birgersson G, Seyoum E, Tekie H, Ignell R, et al. Fresh, dried or smoked? repellent properties of volatiles emitted from ethnomedicinal plant leaves against malaria and yellow fever vectors in Ethiopia. Malar J. 2011; 10: 1-14.
- Seyoum A, Kabiru E, Killeen GF, Hassanali A. Repellency of live potted plants against Anopheles gambiae from human baits in semi-field experimental huts. Am J Trop Med Hyg. 2002; 67: 191-195.
- Patel EK, Gupta A, Oswal RJ. A Review on: Mosquito RepellentMethods. In: Ijpcbs. 2012; 310-317.
- Haris A, Azeem M, Abbas MG, Mumtaz M, Moz?ratis R, Binyameen M. Prolonged Repellent Activity of Plant Essential Oils against Dengue Vector, Aedes aegypti. Molecules. 2023; 28: 1351
- Brown M, Hebert AA. Insect repellents: An overview. J Am Acad Dermatol. 1997; 36: 243-249.
- Antwi FB, Shama LM, Peterson RKD. Risk assessments for the insect repellents DEET and picaridin. Regul Toxicol Pharmacol. 2008; 51: 31-36.
- Imoisi C, Okhale SE. Chemical Composition Analysis of Eucalyptus citriodora Essential Oil Using GC-MS and NMR Spectroscopy. Trends Agric Sci. 2024; 3: 83-90.
- Drapeau J. Insect Repellents Based on para-Menthane-3,8-diol. REGENSBURG; 2017.
- Borrego LG, Ramarosandratana N, Jeanneau E, Métay E, Ramanandraibe VV, Andrianjafy MT, et al. Effect of the Stereoselectivity of para-Menthane-3,8-diol Isomers on Repulsion toward Aedes albopictus. J Agric Food Chem. 2021; 69: 11095-11109.
- Dambolena JS, Zunino MP, Herrera JM, Pizzolitto RP, Areco VA, Zygadlo JA. Terpenes: Natural Products for Controlling Insects of Importance to Human Health - A Structure-Activity Relationship Study. Psyche (London). 2016; 2016: 1-17.
- Liakakou A, Angelis A, Papachristos DP, Fokialakis N, Michaelakis A, Skaltsounis LA. Isolation of Volatile Compounds with Repellent Properties against Aedes albopictus (Diptera: Culicidae) Using CPC Technology. Molecules. 2021; 26: 1-13.
- Nesterkina M, Bernier UR, Tabanca N, Kravchenko I. Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti. Open Chem. 2018; 16: 95-98.
- Pratiwi MAM, Purwati. The Repellent Activity Test of Rosemary Leaf (Rosmarinus officinalis l) Essential Oil Gel Preparations Influence on Aedes aegypti Mosquito. J Phys Conf Ser. 2021; 1788: 012016.
- Singtothong C, Gagnon MJ, Legault J. Chemical composition and biological activity of the essential oil of Amomum biflorum. Nat Prod Commun. 2013;8: 265-267.
- Luo DY, Yan ZT, Che LR, Zhu JJ, Chen B. Repellency and insecticidal activity of seven Mugwort (Artemisia argyi) essential oils against the malaria vector Anopheles sinensis. Sci Rep. 2022; 12: 5337.
- Barnard DR, Bernier UR, Posey KH, Xue R De. Repellency of IR3535, KBR3023, para-menthane-3,8-diol, and deet to black salt marsh mosquitoes (Diptera:Culicidae) in the Everglades National Park. J Med Entomol. 2002; 39: 895-899.
- Mng’ong’o FC, Sambali JJ, Sabas E, Rubanga J, Magoma J, Ntamatungiro AJ, et al. Repellent plants provide affordable natural screening to prevent mosquito house entry in tropical rural settings-results from a pilot efficacy study. PLoS One. 2011; 6: e25927.
- Rana VS, Prasad D, Blazquez MA. Chemical composition of the leaf oil of lantana camara. J Essent Oil Res. 2005; 17: 198-200.
- Liu ZL, Yu M, Li XM, Wan T, Chu SS. Repellent activity of eight essential oils of chinese medicinal herbs to Blattella germanica L. Rec Nat Prod. 2011; 5: 176-83.
- Pavela R. Larvicidal property of essential oils against Culex quinquefasciatus Say (Diptera : Culicidae). 2009; 30: 311-315.
- Omolo MO, Okinyo D, Ndiege IO, Lwande W, Hassanali A. Repellency of essential oils of some Kenyan plants against Anopheles gambiae. Phytochemistry. 2004; 65: 2797-2802.
- Tawatsin A, Asavadachanukorn P, Thavara U, Wongsinkongman P, Bansidhi J, Boonruad T, et al. Repellency of essential oils extracted from plants in Thailand against four mosquito vectors (Diptera: Culicidae) and oviposition deterrent effects against Aedes aegypti (Diptera: Culicidae). Southeast Asian J Trop Med Public Health. 2006; 37: 915-931.
- Guillory JK. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals Edited by Maryadele J. O’Neil, Patricia E. Heckelman, Cherie B. Koch, and Kristin J. Roman. Merck, John Wiley. J Med Chem. 2007; 50: 590.
- Burdock GA. Flavor Ingredients A. 6th ed. Fenaroli’s Handbook of Flavor Ingredients. 2021. 31-150.
- TAKESHITA T, KITAJIMA M. Stereoisomers of 2,3-Camphane Diols*. 1959; 985-991.
- Ramiharimanana FDi, Andrianjafy MT, Ramarosandratana NH, Andrianarijaona TE, Rambala Rakotomena NAH, Metay E, et al. Chirality Effects on Repellent Properties of 4-Alkoxycoumarins Against Asian Tiger Mosquito (Diptera: Culicidae). J Med Entomol. 2022; 59: 430-439.
- Dickens JC, Bohbot JD. Mini review: Mode of action of mosquito repellents. Pestic Biochem Physiol. 2013; 106: 149-155.
- WHO. Guidelines for efficacy testing of mosquito repellents for human skin. Who/Htm/Ntd/Whopes/20094. 2009; 37.
- Ramarosandratana NH, Ralimanana SV, Ranarijaona MM, Métay E, Ramanandraibe V, Andrianjafy MT, et al. Human Age and Sex Influences on the Repellent Activity of PMD Towards Aedes Albopictus. Am J Entomol. 2023; 7: 62-69.
- Yoshinori Shono I, Keisuke Watanabe A, Hiroko Sekihachi T, Akiko Kakimizu N, Masaya Suzuki T, Noritada Matsuo I. MONOTERPENEDIOL INSECT REPELLENTS. 1992; 23: 2073-2076.