Additional Roles of A-MSH or MC1R Signaling in Melanomagenesis Beyond Melanogenesis
- 1. Department of Dermatology, Beijing Chaoyang Hospital, Capital Medical University, China
- 2. Department of Dermatology, Boston University School of Medicine, USA
- 0. Both authors contribute equally
Citation
Dong F, Cui R, Liu F (2013) Additional Roles of Α-MSH/MC1R Signaling in Melanomagenesis Beyond Melanogenesis. J Dermatolog Clin Res 1(1): 1001.
INTRODUCTION
Skin color is determined by epidermal melanin content, the function of which remains poorly understood. Clinically, there is a lower incidence of melanoma in individuals with high levels of constitutive brown/black pigment and/or acquired pigmentation (i.e. tanning). Conversely, individuals with red hair, blue eyes, and inability to tan are at higher risk for developing melanoma. Therefore, pigmentation represents a two-edged sword, with brown/black pigment (eumelanin) being protective and reddish pigment (pheomelanin) predisposing to melanoma [1,2]. However, the lower incidence of melanoma in albinos compared to individuals with red hair/fair skin underscores the complexity of the role of pigmentation in melanoma development. Together, there is essentially one key question of clinical significance to be addressed: why are red-haired individuals so prone to developing melanoma?
The melanocortin-1-receptor (MC1R) is one of the key genes that regulates hair and skin color. MC1R hypomorphic variants are associated with red hair and fair skin in humans. Previous reports demonstrated that three most common variants of MC1R, R151C, R160W, and D294H have a significant association with high melanoma incidence [3-6]. It is well known that melanoma risk attributable to MC1R may arise through the determination of the tanning response of skin to UV light, which can then either ameliorate or exacerbate the genotoxic effects of sunlight [7-9]. However, the connections between MC1R variants and melanoma incidence also identified in darkly-pigmented Caucasians [8], in which MC1R variants are detected from 15% to 33% among dark-haired individuals and to 42% among dark-eyed individuals [8]. Furthermore, MC1R variants are more frequently detected in melanomas with somatic Braf mutation [10-13], suggesting that MC1R variants may have more specific roles in UV-induced mutagenesis. These findings suggest that MC1R signaling pathway may have an additional role in skin carcinogenesis beyond melanogenesis.
α-MSH/MC1R in DNA repair
p53 activation represents a “UV sensor/effector” for skin pigmentation, with its key mechanistic role being the transcriptional activation of POMC [14].The tumor-suppressor protein, p53, plays a central role in the cellular response to UV-induced DNA damage/repair [15].On the other hand, the Abdel-Malek group has demonstrated that α-MSH can prevent UV-induced DNA damage in melanocytes [16-20]. We further demonstrated a novel pigmentation-independent mechanism that underlies MSH-mediated DNA repair following UVB irradiation. We found that α-MSH binds to MC1R and the complex activates adenylate cyclase activity, which in turn activates XPA binding protein 1 (XAB1) that induces nuclear translocation of XPA, a critical factor in controlling nucleotide excision repair (NER) signaling pathways [21] (Figure 1).
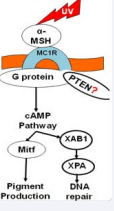
Figure 1 α-MSH/MC1R.
MC1R is a regulator of PTEN after UV exposure
To define the mechanism by which α-MSH/MC1R maintains melanocyte viability, we explored links between α-MSH/MC1R and genes regulated by G protein-coupled receptors (GPCR) using animal models and human subjects because MC1R is a GPCR [22]. We found that PTEN expression in melanocytes was significantly lower among people carrying variant alleles of MC1R compared to baseline pre-treatment levels after UV exposure, suggesting that the MC1R status impacts PTEN expression levels in melanocytes after ultraviolet radiation (UVR) exposure [23]. The PTEN/phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway is crucial to many aspects of cell growth and survival [24]. In this signaling pathway, the product of PI3K, phosphatidylinositol,3,4,5 triphosphate or PIP3, recruits AKT to the plasma membrane via directly interacting with the PH-domain of AKT, where AKT becomes fully activated by other kinases including PDK1 and mTORC2, and in turn exerts its crucial roles by inducing cell proliferation and DNA repair [25]. In melanoma, deletion of the PTEN gene is observed in 30-50% of melanoma cell lines and in 5-20% of primary human melanomas [26]. A recent report showed that abrogation of BRafV600E-induced senescence by PI3K pathway activation (e.g. PTEN deletion) contributes to melanoma development [27]. Recent studies have also shown that UVR causes gene alterations of PTEN in skin cells [28].
To characterize the underlying molecular mechanisms, we tested whether MC1R physically interacts with PTEN. We found that MC1R protein interacts with the PTEN-C2 domain, which protects PTEN from degradation, leading to inhibition of AKT phosphorylation after UV exposure [23].
Given the correlation between melanocyte senescence prevalent in melanocytic nevus and individuals with MC1R variants [29], we next detect senescent melanocytes in shMC1R human primary melanocytes following low-dose UVB exposure (25 J/m2 ). We found that MC1R silencing induced melanocyte senescence [23].
The premature senescent phenotype is observed in nevi under pathological conditions such as BRafV600E expression [30]. This is considered as a natural barrier to prevent oncogeneinduced transformation and in most cases, additional inactivation of the p53/p21 and p16/Rb tumor suppressor pathways is required to bypass this senescent phenotype. To identify the critical role of MC1R in melanoma development, we used genetically engineered human immortal melanocytes (hTERT/ p53DD/CDK4(R24C) [31]. We found that depletion of MC1R is not sufficient to confer cellular transformation in genetically engineered human melanocytes [23]. These results indicate that additional genetic alterations are required for transformation of human melanocytes. To this end, it has been reported by multiple groups that oncogenic BRafV600E mutation is detected in over 60% of human melanomas and is considered a driving force to facilitate melanoma development. Thus, we intend to further investigate the role of BRafV600E in MC1R deficiencyinduced melanoma. We found that genetically engineered human melanocytes with simultaneous depletion of MC1R and ectopic expression of BRafV600E resulted in a robust melanocyte mediaindependent growth in vitro [23].
Finally, correlations between MC1R genetic status and PTEN activity in melanomas were characterized. We found that PTEN mutations are significantly (p < 0.05) more frequently detected in melanomas for individuals with wild-type MC1R than those with MC1R variant alleles [23].
A recent report demonstrated that the MC1R-controled pheomelanin pigment pathway produces ultraviolet-radiationindependent carcinogenic that contributes to melanoma development by a mechanism of oxidative damage [32]. Our studies establish the MC1R-PTEN interaction as a central regulator in response of melanocytes to UV exposure. These two studies are complementary each other for further elucidation of the pigmentary and non-pigmentary mechanisms governing melanoma development. These results will broaden our understanding of melanoma development and help us to devise more effective preventive and therapeutic strategies for this deadly malignancy.









































































































































