Animal Models of Excessive Healing in Cutaneous Wounds
- 1. Department of Molecular, Cell and Systems Biology, University of California Riverside, USA
Abstract
Hypertrophic scars and keloids are disfiguring fibro proliferative disorders that can significantly impair the quality of life of affected individuals. Their treatments are challenging given how little is known about the mechanism underlying their development. The lack of in vivo preclinical animal models has greatly hindered research efforts aimed at improving our understanding of these diseases and developing new therapeutic approaches. This review aims to provide an overview of current preclinical models of hypertrophic scars and keloids in murine, rabbits, porcine, guinea pigs, dogs, and horse. In showing their strengths, limitations, and distinct utilities, we hope that investigators can utilize a model that is appropriate to their research endeavor. In addition, this paper urges researchers to complement their investigations in animal studies with those conducted with clinical samples, a strategy that must be employed until more suitable preclinical models are developed.
Keywords
- Murine
- Rabbit
- Porcine
- Keloids
- Hypertrophic scars
Citation
Saeed S, Martins-Green M (2022) Animal Models of Excessive Healing in Cutaneous Wounds. J Dermatolog Clin Res 10(2): 1149.
ABBREVIATIONS
HS: Hypertrophic Scars; ECM: Extracellular Matrix; SSc: Systemic Sclerosis; TGF-β: Transforming Growth Factor Beta; CTGF: Connective Tissue Growth Factor; Akt: Protein Kinase B; CAPN2: Calpain-2; SEI: Scar Elevation Index; TGFβ1: Transforming Growth Factor Beta 1; COL1α1: Type 1 Collagen Alpha 1; KL: Keloid Lesion; IL-1β: Interleukin-1β; TGF-β2: Transforming Growth Factor-Beta 2; EGT: Exuberant Granulation Tissue.
INTRODUCTION
Cutaneous wound healing is an essential physiological process that re-establishes the skin barrier and repairs damaged tissue following an injury. Whereas a wound may successfully heal under optimal circumstances, interruptions of the sequential processes involved in healing by local and systemic factors can derail proper progression, temporally and spatially, and lead to impaired wound healing or even non-healing.
Although the exact physiological processes that underlie excessive healing remain poorly understood, recent investigations have yielded significant information on factors that affect the excessive healing process: upregulation of genes that encode for pro-inflammatory cytokines in the dermis causes chronic inflammation in the wound tissue, resulting in excessive fibroblast proliferation and extracellular matrix (ECM) deposition [1], as well as abnormal vascularization [2]. Patients with these conditions experience a lower quality of life due to restrictions in mobility, poorer appearance, and psychological impairments such as depression and loss of self-confidence [3].
Incidence of hypertrophic scarring following surgery is roughly 60% [4], with the highest rate occurring following full-thickness burn injuries [5]. Individuals of African descent
are significantly more susceptible to keloids, with occurrences ranging from 6-16% [6].
Hypertrophic scars often develop in areas of high tension (such as the shoulder, knee, and ankle) commonly following a burn injury, are hard in texture, appear red or pink, remain within the wound margins, and tend to regress [7]. They are raised up to 4 mm from the wound bed and contain primarily type III collagen [8,9]. They also have nodules containing myofibroblasts and mucopolysaccharide [6].
Unlike hypertrophic scars, which remain within the wound margins and tend to regress, keloids extend beyond the wound margin and rarely subside [10]. Keloids tend to occur in areas of excisions and appear shiny with brown or purple coloration that may reflect hyperpigmentation [6]. They protrude prominently from the surrounding skin and are composed of irregularly branched and abnormally thick type I and III collagen bundles with no myofibroblast-containing nodules [6,8].
Given the etiological differences between hypertrophic scars and keloids, recent studies have developed distinct animal models to better represent their respective pathophysiology. At the same time, however, major differences between animal skin anatomy and its immune system with that of humans continue to make it difficult to identify the factors responsible for the formation of hypertrophic scars and keloids in humans. In addition, genetic predisposition plays an active role in the development of both healing types, yet this characteristic cannot be considered in animal models except for, potentially, in the red Duroc pig. Hence, such disparities must be recognized when drawing conclusions from experiments using these animal models.
This review aims to present the common and most promising models of excessive healing wounds—both hypertrophic scars
and keloids—developed in murine, rabbits, pigs, guinea pigs, and dogs with the hope that future studies will continue to improve and develop new models (Figure 1).
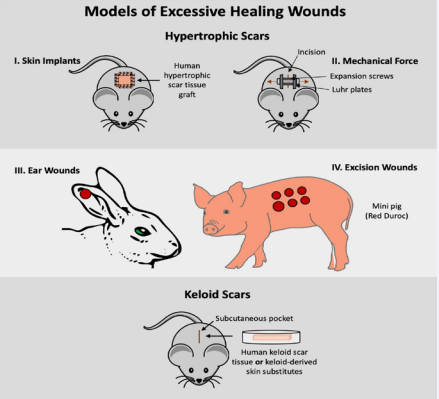
Figure 1 Visual Overview of Models of Excessive Healing Wounds.
Hypertrophic scar models
Normal wounds heal without excessive scarring. Several strategies have been developed to bring about excessive healing, which leads to hypertrophic scars (HS). These strategies include the use of genetically modified animal strains, repeated application of mechanical force, burn injuries, and the rabbit ear, which has a natural propensity to over-scarring (Table 1).
|
Table 1: Models for Excessive Wound Healing Studies. |
|||||
|
Type of scar |
Animal |
Type of model |
Overview |
Limitations |
Use |
|
Hypertrophic Scars |
Murine |
Skin grafts |
Involves transplant of human-hypertrophic scar tissue in full-thickness excision wounds generated in immunodeficient mice. Tissue may also be transplanted in sandwich- island flap. |
cannot be studied in nude mice |
|
|
|
|
Mechanical loading |
Involves delivery of force on linear incision wounds in immunocompetent and/or knockout mice strain |
|
|
|
|
Rabbit |
Ear wound |
Involves full-thickness excision wounds on rabbit ear that extended to cartilage |
of human hypertrophic scar development |
|
|
|
Porcine |
Excision wound |
Involves full-thickness excision wounds on dorsum of Red Duroc or Mini Bama pig |
costly
|
|
|
|
|||||
|
|
|||||
|
|
|||||
|
Keloid |
Murine |
Keloid transplant Engineered tissue implants |
Involves inserting human- keloid scar tissue into the subcutaneous pocket of immunodeficient mice or sandwich-island flap in rat. Involves inserting keloid- derived skin substitutes in a subcutaneous pouch or on full-thickness excision wounds in immunodeficient mice |
|
|
|
Equine |
Exuberant granulation tissue (EGT) development |
Studies involve comparing Equine EGT to human keloid tissue. |
|
|
|
Pre- clinical relevance of these models should be assessed by their morphological and histological similarities to human HS. These scars contain primarily rounded whorls of immature collagen consisting of mostly-type III collagen fibrils, small blood vessels, abundant mucopolysaccharide, and nodules that express alpha- smooth muscle actin and contain myofibroblast [9,11].
Mouse and Rat models
Amongst the earliest development of a possible HS model in mice, investigators created full-thickness 2 cm2 excision wounds on a tight-skin mouse (Tsk/+) strain [12]. Because Tsk/+ mice have a hypodermis that is firmly adherent to the underlying muscle tissue, they are very similar to human skin and do not contract during wound healing [12]. Aside from the noted delayed wound closure in the Tsk/+ mice, histological analysis also showed hyperplastic granulation tissue under the epidermal layer. In addition, swirls of collagen fibrils were apparent, as is the case in human HS [12,13]. While the Tsk/+ mouse strain shows
fibrosis that is prevalent in HS, the model still fails to properly recapitulate the pathophysiological process. Nevertheless, the model has been extensively used for studies on systemic sclerosis (SSc), an autoimmune disease that develops excessive fibrosis in many different organs and tissues and causes alterations in the microvasculature and inflammatory response [14-16].
Another model for hypertrophic scars was created by transplanting full-thickness human skin grafts onto full- thickness excision wounds created on the dorsum of athymic nude mice [17]. Grafts turn black, shedding the epidermis and the upper portion of the dermis approximately 1 month after the transplant and before forming hypertrophic scars. Gross observation showed redness and thickening of the grafted skin, while histological examination showed dense and disorganized collagen fibers, all characteristics of hypertrophic scars [17]. Interestingly, partial- thickness human skin engrafted onto full- thickness excisions in athymic nude mice developed more scar than full-thickness grafts and showed increased expression of Transforming growth factor beta (TGF-β), connective tissue growth factor (CTGF), type I collagen, and heat shock protein 47, all indicators of fibrosis [18]. When investigators harvested skin biopsies of the nude mice grafted with partial-thickness human skin, they found that the biopsies were histologically, immune- histochemically, and morphologically consistent with human hypertrophic scars [19] this was further validated by other groups [18]. The increased scar formation from partial- thickness grafts compared to full-thickness grafts may be attributed to how the tissue itself is prepared. The use of a dermatome may initiate an injury response in the skin, and this could be responsible for the increased expression of proinflammatory cytokines and profibrotic growth factors in partial-thickness compared to full-thickness skin grafts. Consequently, this enhances the recruitment of leukocytes and local fibroblast activities. Similar observations have been made in the clinic whereby wounds covered with partial-thickness skin grafts exhibit greater fibrosis and contraction than those with full-thickness grafts [19].
A burn model was developed in immune-competent mice to specifically study hypertrophic scar development, a painful condition experienced by about 67% of burn injury victims [5,20]. This model showed many phenotypic similarities to human HS [20]. Third-degree burns were generated on the dorsum of C57BL/6 mice and three days later, were excised and grafted with ear skin. By day 14, skin grafts were significantly contracted and had increased vascular density, granulation tissue, macrophages and mast cell populations, and collagen maturation as measured by immunohistochemically and histology studies. Furthermore, skin grafts had the same elasticity as human HS.
Yet other thermal burn models to evaluate HS formation used two different methods in rats: (i) thermal burns created via a 1470 nm laser light and (ii) chemical burns created through topical application of sodium hydroxide (NaOH). Excision wounds created using a punch biopsy were used for comparison with normal healing. All groups showed an extensive increase in fibroblast and collagen fibers that were denser and more oriented than normal skin [21].
However, the chemical burn model, while having the largest initial lesion size and least axial damage along tissue depth following wounding, formed skin that was twice as thick as that in the thermal burn and punch biopsy wounds [21]. Based on these observations, the investigators suggested that shallow but wide wounds may help maximize collagen generation after healing.
Other models of hypertrophic scars have been developed in immune-competent mice by mechanical loading. In one such study, tension was applied on 2 cm linear incision wounds created in C57BL/6 mice via a mechanical loading device constructed of expansion screws and Luhr plate supports [22]. The resulting
scars showed near-identical histopathology to that of human HS with dramatic increases in volume and cellular density. This was accompanied by a decrease in cellular apoptosis and increased activation of the pro-survival marker Akt. Akt is an important mediator in the transition between the proliferative and remodeling phase of wound healing by promoting cell survival, migration, and collagen production. Among its pathways, Akt accomplishes this by increasing anti-apoptotic BclII and inhibiting p53-mediated apoptosis. To clarify the role of apoptosis in HS development, wounds were generated in mice with altered apoptotic pathways. Wounds on proapoptotic BclII-null mice displayed reduced cellular density and scar hypertrophy. Conversely, in p53-null mice with downregulated cellular apoptosis, wounds showed significantly greater cellular density and scar hypertrophy. These results suggest that early mechanical loading promotes HS formation by inhibiting cellular apoptosis through Akt-dependent pathways [22]. Full-thickness excisions generated on the dorsum of CXCR3 knockout mice also resembled many aspects of human hypertrophic scars [23]. During the healing process, two chemokines IP-9 and IP-10 bind to the chemokine receptor CXCR3. Signaling through this receptor blocks growth factor-induced motility in fibroblast and endothelial cells by suppressing activation of Calpain-2 (CAPN2). At the same time, IP-9 promoted keratinocyte migration through its activation of calpain. Researchers utilized mice lacking either CXCR3 or the IP-9 ligand as models to evaluate maturation of excisional wounds in the absence of this signaling pathway. Wounds from the knockout mice showed thick scarring, compared to negligible scarring in wild-type mice, in addition to lower tensile strength likely due to the disorganized alignment of collagen fibers and an increase in collagen I, collagen II, and decorin. Histological analysis revealed thickening of the epidermis and dermis; the dermis showed long and thick collagen fibers and excessive collagen content [23].
Recently, a novel model of hypertrophic scar development in the rat tail was developed that showed considerable similarities with human hypertrophic scarring. An excisional wound was created using a scalpel and iris scissors on the tail of rats and the wounds were exposed to mechanical stretching by wrapping the tail around a steel ring. Rat tails left over 24 weeks showed significantly increased scar area, scar height, vessel density, distorted collagen arrangement, and expression of molecules related to hypertrophic scarring [24].
All these models have their merits and limitations. For example, immune deficient models, although very informative, do not provide information within an immune background. The knockout mice are valuable and can provide important mechanistic information, but it is the absence of a molecule, rather than the presence of a molecule. The rat tail model does not have a dermis, which is representative of most areas of the human skin, but it is nonetheless useful for studies of keratinocyte migration and wound closure. Therefore, much work needs to be done to develop a mouse model that more closely mimics HS in humans.
Rabbit models
An important model of hypertrophic scars was developed by Mustoe’s laboratory and continues to find much success to
date. It involves using a trephine to create 6-mm diameter full- thickness excision wounds on the ventral surface of the rabbit ear, that extends to the bare cartilage, followed by covering of the wound with an occlusive polyurethane dressing for 12 days post- wounding. To quantify scarring, the group also developed a Scar Elevation Index (SEI), a ratio of the scar height over that of the surrounding skin [25]. This model was subsequently improved by creating 7-mm diameter wounds as opposed to the previous 6-mm, to show much more scarring [26]. Unpublished data from the group showed that 5-mm wounds failed to generate HSs, and 6-mm wounds appear to be less hypertrophic than 7-mm wounds due to faster epithelialization [26]. Moreover, whereas it was originally postulated that removal of the perichondrium is primarily responsible for the delayed re-epithelization and persistence of elevated hypertrophic scars, a subsequent study omitted excision of the perichondrium and used a 10-mm punch biopsy to create more extensive hypertrophic scars [27]. These wounds showed high similarities to human hypertrophic scars, had a higher SEI, and occurred as early as 3-4 weeks, persisting for more than 60 days [26]. The scars appeared as firm papules, and histological evaluation showed excessive accumulation of horizontally arranged collagenous fibers, inflammatory cells, and increased vascularization, all of which are characteristic of human hypertrophic scars [28]. Furthermore, omitting excision of the perichondrium facilitates not only the development of hypertrophic scars but also reduces the risk of cartilage desiccation and wound infection [27].
The great success of Mustoe’s model can be attributed to its widespread implementation in studies of hypertrophic scars, mostly aimed at understanding the effects of various therapies on hypertrophic scar development. Their model has been repeatedly used to test the efficacy of potential treatments of hypertrophic scars, including inhibitors [29,30], growth factors [31], stem cells [32,33], and radiation [34].
Despite the advantages of the rabbit ear, it must be noted that the resulting healing occurs on an avascular cartilage base [35], which is contrary to the normal wound healing environment in human skin. Nonetheless, given the reproducibility and relative ease of generating hypertrophic scars, the rabbit ear remains an effective model for studying the pathogenesis of hypertrophic scar formation and for evaluating anti-fibrotic therapies.
Porcine models
Recently, researchers developed hypertrophic scars in the red Duroc pig by creating wounds of 8 x 8 cm dimensions on the dorsum of the pig using a dermatome [36]. Hair growth and pigmentation are similar to that of humans as are several histological aspects, such as an absence of elastin and the presence of collagen that is disorganized [36]. However, although significant scarring was observed, it did not resemble some of the visual aspects of human hypertrophic scarring. For example, human hypertrophic scars are red and have abruptly raised edges, whereas those in the Duroc pig were not red and had gradual edges. Although no true collagen nodules were seen in these studies using young pigs, a subsequent study reported the formation of distinct nodules in 5-month-old Duroc pigs [37]. This is probably because young age skin is more regenerative than scarring. Other investigators have further validated the
model, noting increased numbers of myofibroblasts, mast cells, microvessel density, collagen nodules, area of the scars, and TGF-β1 mRNA and protein, as is seen in humans [37,38]. In the case of microvasculature, a comparison between hypertrophic scars developed in the red Duroc pig and those in the Yorkshire pig showed that the latter have significantly less microvasculature and do not mimic human hypertrophic scars [38]. Moreover, it is suggested that shallow wounds (those created with a dermatome setting of 0.015″ to 0.030″) are markedly different from deep wounds (created with the setting of 0.045″ to 0.060″). Indeed only deep wounds resembled human hypertrophic scars [39].
A recent study compared hypertrophic scarring in the red Duroc pig with that in the Guangxi Mini Bama pig and observed similar trends in shape, epidermal thickening, and collagen deposition [40]. The smaller size of the mini pig may make it advantageous over the Duroc pig, serving as a possible replacement. Moreover, an earlier study that used Bama mini- pigs as a model for hypertrophic scars, inflicted full-thickness burn wounds extending through the dermis, as opposed to excision wounds [41]. Hypertrophic scars formed seven weeks after the burn and were characterized as dark purple, raised, firm, increased epidermal and dermal thickness, dense fibroblast populations, and excessive collagen deposition [41], characteristics present in burned patients. The Bama mini pig thus warrants further investigation as a possible model of hypertrophic scars.
Guinea Pig and Dog models
In addition to those in mice, rats, rabbits, and pigs, an HS model has also been developed in albino guinea pigs. Amongst the various procedures tested, a research group showed that some guinea pigs with excisional wounds treated with coal tar followed by removal of the panniculus carnosus developed hypertrophic scarring [42]. Scars were considered hypertrophic given that they presented histological and clinical features similar to those of human hypertrophic scars, such as erythema and scar elevation. However, this guinea pig model lacked consistency in HS development given that only one-third of animals developed HS [42]. In addition, 20% of the animals died during treatment with coal tar given its toxicity.
Another model uses hairless dogs to create a model of HS. Full-thickness excision wounds were generated on the lumbar region of hairless dogs [43]. Compared to dogs with hair, these animals showed the typical characteristics of HS: presenting thicker scars with hypervascularization and more fibroblasts and inflammatory cells, in addition to higher collagen organization and collagen nodules [43,44]. Repaired skin showed hyperpigmentation and thickening of the epidermis and stratum corneum. Unlike the model in red Duroc pigs, wounds showed more erythematous and prominent elevation above the wound margin [43].
KELOID MODELS
Mouse models
Transplant of keloid tissue: A promising keloid mouse model was first developed by transplanting keloid tissues from humans into a subcutaneous pocket made by a 1 cm incision down to the panniculus carnosus on the dorsum of athymic nude mice [45]. At various times ranging from 7 to 60 days post-transplantation, the keloid tissue was removed from the mice and examined microscopically. No changes were observed in morphology when compared to the original keloid specimen. Furthermore, both the original keloid tissue source and the transplanted keloid tissue had similar distributions of glycosaminoglycans, specifically chondroitin-4-sulfate and hyaluronic acid levels [45]. A subsequent report confirmed that no rejection occurred in this model even after 246 days and vascular anastomosis between the host and the transplanted tissue was observed [46]. The authors suggested that 16 days after transplantation was the best time to conduct experiments on this model [46]. In an initial study, penicillamine, acetylcysteine, colchicine, and triamcinolone acetonide were administered to the keloid transplants using this model [47]. The authors noted initial growth of the keloid tissue reaching a peak in growth at 4 weeks post-treatment followed by a regression in size. Following the regression, no difference was observed between the control and experimental groups [47]. Although the use of athymic nude mice for modeling keloids is problematic given that it is well-known that the immune system plays a significant role in excess wound healing, this model may be used to explore treatments that could reduce and/or abolish the keloid tissue.
More recently, a group of investigators reported a very promising model in which human- keloid tissue was transplanted into a subcutaneous pocket created between the panniculus carnosus and the anterior rectus sheath, a fascia anterior to the rectus muscle, of immune- competent mice [48]. Surprisingly, the fibrotic nature and volume of the keloid transplants remained preserved for the entirety of the four-month experiment [48]. Unfortunately, the authors provided very few experimental details, making reproducibility difficult for other groups.
Implants of engineered keloid-like tissue: Stem cells were isolated from the dermal layer of keloids (keloid-derived precursor cells), placed in hydrogel carriers, and implanted subcutaneously into the dorsum of immunocompromised mice [49]. The results showed the development of keloid-like benign tissue that displayed aggressive dermal growth resembling that of tumors [49]. This model, however, lacks keloid epidermal cells and thus does not have dermal-epidermal interactions. In contrast, another model was developed with the presence of both keloid dermal and epidermal cells [50]. These investigators grafted skin substitutes composed of six combinations of keloid-derived cells including normal keratinocytes, keloid keratinocytes, normal fibroblasts, deep keloid fibroblasts, and superficial keloid fibroblasts into athymic nude mice. Skin- substitutes containing deep or superficial keloid fibroblasts showed abnormal organization of collagen fibers, whereas those containing keloid keratinocytes and deep keloid fibroblasts showed thicker skin and elevated expression of type 1 collagen alpha 1 (COL11) [50]. However, macroscopic examination of the grafted-substitute tissue failed to show morphological features of human keloid tissues.
Another model involved the implantation of cultured human keloid fibroblasts into subcutaneous pouches of athymic nude mice [51]. Before implantation, keloid fibroblasts were
transferred to a poly (lactic-co-glycolic acid) copolymer and cultured in a rotary cell culture system for one week. The control group was implanted with only the copolymer and the implants were collected 30-180 days after surgery. At collection, the weight and size of the implants continued to increase as opposed to the control group, which decreased in size. Histological staining of the “keloid” implants showed that the human keloid fibroblasts were still present, and the collagen had the characteristics of keloids. Fibroblasts in the implants also had elevated levels of rough endoplasmic reticulum, as is seen in human keloids [51]. Similar procedures were used to create another promising model of keloids [52]. Human keloid fibroblasts were seeded on sponge- like scaffolds, allowed to populate the sponges in a rotary cell culture system, and then implanted subcutaneously in athymic nude mice. Given the excessive deposition of collagen fibrils in keloids, an inhibitor of collagen fibril formation was injected into the constructs and resulted in a significant decrease in collagen content [52].
Another model was developed in which a porous polyethylene ring that supported a keloid-derived epidermal-dermal skin substitute was implanted on the dorsum of athymic nude mice [53]. These investigators showed that the model contained key features of keloid tissue, including nodular morphology, increased and abnormal collagen organization, epidermal hyperplasia, and disrupted dermal-epidermal junctions [53].
Other investigators using nude mice, have focused more on recreating distinctive features of keloids, such as the presence of excessive deposition of versican [54]. Mesenchymal cells derived from keloid lesions showed elevated versican production in culture. Hence, keloid lesion (KL) cells or normal fibroblasts (as control) were seeded in collagen sponges and implanted subcutaneously in the mouse. After one month, both sponges were retrieved, and their weights were compared. Sponges seeded with KL cells presented significantly greater weight increase than those seeded with normal fibroblast. Furthermore, IL-1β, shown to suppress the expression of versican genes in vitro, was injected in KL-seeded sponges. These sponges showed reduced implant growth, showing an apparent therapeutic potential of IL-1β in inhibiting keloid formation [54]. Hence, this model has promise for investigations into treatments for versican0- containing keloids.
There are several limitations of the skin-transplant model for keloid studies. First, transplanted human keloids are often older and more mature tissue because only freshly excised keloid tissue can be used. This, consequently, does not allow for modeling of the early keloid development [55]. Second, transplanted keloid tissue is usually devoid of the epidermis therefore, dermal- epidermal interactions cannot be investigated [55]. Third, since immunodeficient models must be used for the transplants to prevent rejection, immune system involvement in the process of keloid development cannot be studied [55].
Rat models
The small size and short lifespan of the nude mouse, in addition to the necessity of using only small transplants and biopsies, make the nude mouse model not optimal for studying keloid biology. This limitation was overcome by the development of a breeding colony of athymic nude rats [56], followed by studies ascertaining the feasibility of human skin transplants in these rats. Soon after, it was reported that these nude rats could be used in developing and maintaining hypertrophic scars and keloids [57]. An improved model was described whereby a superficial inferior epigastric pedicle flap was generated and human-keloid or hypertrophic scar specimens were sutured on the inner surface of flap [58]. Three weeks later, a sandwich-island flap was created where the flap was raised and isolated with its pedicle while a catheter was placed on the flap and sandwiched by it to allow for the subcutaneous application of substances. The flap was then tunneled to the ipsilateral flank and isolated from the surrounding host tissue. The flap’s isolated pedicle and its position separate from the surrounding host tissue guaranteed the survival of the explant. Histological characteristics of fibroblasts and epithelial cells of the explanted tissue remained unchanged and the disorganized pattern of collagen bundles, typical of keloids and hypertrophic scars, remained visible for up to 12 months [58]. At the same time, however, culture of fibroblasts derived from both hypertrophic scars and keloid explants showed less aggressive growth as compared to those from the original specimen [58]. This model was used to perfuse TGF-β2 or anti-TGF-β2 antibodies via the catheter [59]. Results from immunohistochemical analyses showed that there was increased production of endogenous TGF- β2, collagen I, and collagen III following perfusion of exogenous TGF-β2 but the opposite occurred with perfusion of anti-TGF-β2 antibody [59].
Equine Exuberant Granulation Tissue Development Model: Wounding in horses often involves the loss of large amounts of tissue that require healing with second intention which relies on granulation tissue development followed by re- epithelialization and wound contraction [60]. This makes them susceptible to ulceration and fibro proliferative conditions, such as exuberant granulation tissue (EGT) development that occurs on the distal limb of horses. Due to the high frequency of extensive tissue loss and contamination at this location, these wounds must heal by second intention. However, a persistent yet weak inflammatory response, in addition to local hypoxia and ischemia, contributes to the development of EGT [60]. It should also be noted that the only known mammals to naturally develop excessive granulation tissue during wound healing are humans and horses [61]. Equine EGT resembles human keloids both grossly and histologically: fibro proliferation extends beyond the margins of the wound and appears raised, they behave clinically like benign tumors, there is occlusion of the microvasculature, and there is an overproduction of extracellular matrix and in particular collagen deposition [61,62]. A comparison of equine EGT and human keloid tissues showed disorganized collagen fibers (although it was not the standard keloid-collagen arrangement), increased fibroblast populations, and minimal vascularity. However, EGT tissue has a more pronounced inflammation with increases in myofibroblasts and polymorph nuclear cells and delayed re-epithelialization and wound closure [60,61]. Nevertheless, the equine EGT model certainly warrants more attention as a possible model for keloids.
CONCLUSION
There is no doubt that animal models are a critical component in biological research since they provide investigators with a stepping-stone through which in vitro and in vivo studies in model organisms may be translated to clinical research. However, this necessitates that animal models be created in ways that render them clinically relevant. Although there has been much progress in the development of animal models of wound healing, significant advancements are still required to be made in models that replicate excessive healing wounds, especially keloids.A critical obstacle in the development of appropriate models is a lack of reproducibility.
For consistent studies to occur, researchers must describe protocols in detail and pay close attention to established guidelines. Lastly, we recommend that forthcoming models of excessive healing wounds—or of any type of wound for that matter—be scrutinized and their similarity with human wounds be compared before ensuing publication [63]. This will ensure that experimental data is clinically relevant, ultimately facilitating the development of treatments for impaired healing wounds.
ACKNOWLEDGEMENTS
This work was by the UCR Chancellor’s Research Fellowship, and a UCR mini-research grant to S. Saeed. It was also supported by 1 R21 AI156688-01 to M. Martins-Green.
REFERENCES
- Ogawa R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int J Mol Sci. 2017; 18: 3.
- Amadeu T, Braune A, Mandarim-de-Lacerda C, Porto LC, Desmoulière A, Costa A. Vascularization pattern in hypertrophic scars and keloids: a stereological analysis. Pathol Res Pract. 2003; 199: 469–73.
- Bock O, Schmid-Ott G, Malewski P, Mrowietz U. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006; 297: 433.
- Mahdavian Delavary B, van der Veer WM, Ferreira JA, Niessen FB. Formation of hypertrophic scars: Evolution and susceptibility. J Plast Surg Hand Surg. 2012; 46: 95–101.
- Bombaro KM, Engrav LH, Carrougher GJ, Wiechman SA, Faucher L, Costa BA, et al. What is the prevalence of hypertrophic scarring following burns? Burns. 2003; 29: 299– 302.
- Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic Scarring and Keloids: Pahomechanisms and Current and Emerging Treatment Strategies. Mol Med. 2011; 1: 113–25.
- Schmieder SJ, Ferrer-Bruker SJ. Hypertrophic Scarring. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020.
- Ehrlich HP, Desmoulière A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994; 145: 105– 13.
- Hawkins HK, Jay J, Finnerty CC. 46 - Pathophysiology of the Burn Scar. In: Herndon DN, editor. Total Burn Care (Fifth Edition) [Internet]. Elsevier; 2018; 466-475.e3.
- Tripathi S, Soni K, Agrawal P, Gour V, Mondal R, Soni V. Hypertrophic scars and keloids: a review and current treatment modalities. Biomed Dermatol. 2020; 4: 11.
- Larson BJ, Nauta A, Kawai K, Longaker MT, Lorenz HP. 3 - Scarring and scarless wound healing. In: Farrar D, editor. Advanced Wound Repair Therapies [Internet]. Woodhead Publishing; 2011; 77–111.
- Ehrlich HP, Needle AL. Wound healing in tight-skin mice: delayed closure of excised wounds. Plast Reconstr Surg. 1983; 72: 190–8.
- Seo BF, Lee JY, Jung SN. Models of Abnormal Scarring. BioMed Res Int [Internet]. 2013 [cited 2020 Dec 31]; 2013.
- Artlett CM. Animal models of systemic sclerosis: their utility and limitations. Open Access Rheumatol Res Rev. 2014; 6: 65–81.
- Long KB, Artlett CM, Blankenhorn EP. Tight skin 2 mice exhibit a novel time line of events leading to increased extracellular matrix deposition and dermal fibrosis. Matrix Biol. 2014; 38: 91–100.
- Pablos J, Everett E, Norris J. The tight skin mouse: An animal model of systemic sclerosis. Clin Exp Rheumatol. 2004; 22: S81-5.
- Yang D, Li S, Wu J, Chen Y, Li G, Bi S, et al. Establishment of a Hypertrophic Scar Model by Transplanting Full-Thickness Human Skin Grafts onto the Backs of Nude Mice. Plast Reconstr Surg. 2007; 119: 104–9.
- Wang J, Ding J, Jiao H, Honardoust D, Momtazi M, Shankowsky HA, et al. Human hypertrophic scar-like nude mouse model: Characterization of the molecular and cellular biology of the scar process. Wound Repair Regen. 2011; 19: 274–85.
- Momtazi M, Kwan P, Ding J, Anderson CC, Honardoust D, Goekjian S, et al. A nude mouse model of hypertrophic scar shows morphologic and histologic characteristics of human hypertrophic scar. Wound Repair Regen. 2012; 21: 77–87.
- Ibrahim MM, Bond J, Bergeron A, Miller KJ, Ehanire T, Quiles C, et al. A novel immune competent murine hypertrophic scar contracture model: A tool to elucidate disease mechanism and develop new therapies. Wound Repair Regen. 2014; 22: 755–64.
- Kim M, Kim H, Kang HW. Comparative evaluations of hypertrophic scar formation in in vivo models. Lasers Surg Med. 2018; 50: 661–8.
- Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007; 21: 3250–61.
- Yates CC, Krishna P, Whaley D, Bodnar R, Turner T, Wells A. Lack of CXC Chemokine Receptor 3 Signaling Leads to Hypertrophic and Hypercellular Scarring. Am J Pathol. 2010; 176: 1743–55.
- Zhou S, Wang W, Zhou S, Zhang G, He J, Li Q. A Novel Model for Cutaneous Wound Healing and Scarring in the Rat. Plast Reconstr Surg. 2019; 143: 468–77.
- Morris DE, Wu L, Zhao LL, Bolton L, Roth SI, Ladin DA, et al. Acute and Chronic Animal Models for Excessive Dermal Scarring: Quantitative Studies. Plast Reconstr Surg. 1997; 100: 674–81.
- Kloeters O, Tandara A, Mustoe TA. Hypertrophic scar model in the rabbit ear: a reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen. 2007; 15: S40–5.
- Jia S, Zhao Y, Mustoe TA. The effects of topically applied silicone gel and its silver derivative on the prevention of hypertrophic scarring in two rabbit ear-scarring models. J Plast Reconstr Aesthet Surg. 2011; 64: e332–4.
- Nabai L, Ghahary A. Hypertrophic Scarring in the Rabbit Ear: A Practical Model for Studying Dermal Fibrosis. In: Rittié L, editor. Fibrosis: Methods and Protocols [Internet]. New York, NY: Springer; 2017; 81–9.
- Ko J, Kim P, Zhao Y, Hong S, Mustoe T. HMG-CoA Reductase Inhibitors (Statins) Reduce Hypertrophic Scar Formation in a Rabbit Ear Wounding Model. Plast Reconstr Surg [Internet]. 2012; 129: 252e-261e.
- Xu W, Hong SJ, Zeitchek M, Cooper G, Jia S, Xie P, et al. Hydration Status Regulates Sodium Flux and Inflammatory Pathways through Epithelial Sodium Channel (ENaC) in the Skin. J Invest Dermatol. 2015; 135: 796–806.
- Xie JL, Bian HN, Qi SH, Chen HD, Li HD, Xu YB, et al. Basic fibroblast growth factor (bFGF) alleviates the scar of the rabbit ear model in wound healing. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2008; 16: 576–81.
- Chu H, Wang Y, Wang X, Song X, Liu H, Li X. Effects of transplanted adipose derived stem cells on the expressions of α-SMA and DCN in fibroblasts of hypertrophic scar tissues in rabbit ears. Exp Ther Med. 2018; 16: 1729–34.
- Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG. Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther [Internet]. 2015; 6: 145.
- Brown R, Lee M, Sisco M, Kim J, Roy N, Mustoe T. High-Dose Ultraviolet Light Exposure Reduces Scar Hypertrophy in a Rabbit Ear Model. Plast Reconstr Surg. 2008; 121: 1165–72.
- Davidson JM, Yu F, Opalenik SR. Splinting Strategies to Overcome Confounding Wound Contraction in Experimental Animal Models. Adv Wound Care. 2013; 2: 142–8.
- Zhu KQ, Engrav LH, Gibran NS, Cole JK, Matsumura H, Piepkorn M, et al. The female, red Duroc pig as an animal model of hypertrophic scarring and the potential role of the cones of skin. Burns. 2003; 29: 649–64.
- Harunari N, Zhu KQ, Armendariz RT, Deubner H, Muangman P, Carrougher GJ, et al. Histology of the thick scar on the female, red Duroc pig: Final similarities to human hypertrophic scar. Burns J Int Soc Burn Inj. 2006; 32: 669–77.
- Xie Y, Zhu KQ, Deubner H, Emerson DA, Carrougher GJ, Gibran NS, et al. The Microvasculature in Cutaneous Wound Healing in the Female Red Duroc Pig Is Similar to That in Human Hypertrophic Scars and Different From That in the Female Yorkshire Pig. J Burn Care Res. 2007; 28: 500–6.
- Zhu KQ, Engrav LH, Tamura RN, Cole JA, Muangman P, Carrougher GJ, et al. Further similarities between cutaneous scarring in the female, red Duroc pig and human hypertrophic scarring. Burns. 2004; 30: 518–30.
- Ning X, Yang K, Shi W, Xu C. Comparison of hypertrophic scarring on a red Duroc pig and a Guangxi Mini Bama pig. Scars Burn Heal. 2020; 6.
- Deng X, Chen Q, Qiang L, Chi M, Xie N, Wu Y, et al. Development of a Porcine Full- Thickness Burn Hypertrophic Scar Model and Investigation of the Effects of Shikonin on Hypertrophic Scar Remediation. Front Pharmacol. 2018; 9: 590.
- Aksoy MH, Vargel I, Canter IH, Erk Y, Sargon M, Pinar A, et al. A New Experimental Hypertrophic Scar Model in Guinea Pigs. Aesthetic Plast Surg. 2002; 26: 388–96.
- Kimura T. Hairless Descendants of Mexican Hairless Dogs: An Experimental Model for Studying Hypertrophic Scars. J Cutan Med Surg. 2011; 15: 329–39.
- Rössler S, Nischwitz SP, Luze H, Holzer-Geissler JCJ, Zrim R, Kamolz LP. In Vivo Models for Hypertrophic Scars—A Systematic Review. Medicina (Kaunas). 2022; 58: 736.
- Shetlar MR, Shetlar CL, Hendricks L, Kischer CW. The use of athymic nude mice for the study of human keloids. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1985; 179: 549–52.
- Kischer CW, Sheridan D, Pindur J. Use of nude (athymic) mice for the study of hypertrophic scars and keloids: vascular continuity between mouse and implants. Anat Rec. 1989; 225: 189–96.
- Waki EY, Crumley RL, Jakowatz JG. Effects of Pharmacologic Agents on Human Keloids Implanted in Athymic Mice: A Pilot Study. Arch Otolaryngol Head Neck Surg. 1991; 117: 1177–81.
- Park TH, Rah DK, Chang CH, Kim SY. Establishment of Patient-Derived Keloid Xenograft Model. J Craniofac Surg. 2016; 27: 1670–3.
- Zhang Q, Yamaza T, Kelly AP, Shi S, Wang S, Brown J, et al. Tumor- Like Stem Cells Derived from Human Keloid Are Governed by the Inflammatory Niche Driven by IL-17/IL- 6 Axis. PLoS One. 2009; 4: e7798.
- Supp DM, Hahn JM, Glaser K, McFarland KL, Boyce ST. Deep and Superficial Keloid Fibroblasts Contribute Differentially to Tissue Phenotype in a Novel In Vivo Model of Keloid Scar: Plast Reconstr Surg. 2012; 129: 1259–71.
- Wang H, Luo S. Establishment of an Animal Model for Human Keloid Scars Using Tissue Engineering Method. J Burn Care Res. 2013; 34: 439–46.
- Chung HJ, Steplewski A, Chung KY, Uitto J, Fertala A. Collagen Fibril Formation. A New Target to Limit Fibrosis. J Biol Chem. 2008; 283: 25879–86.
- Lee YS, Hsu T, Chiu WC, Sarkozy H, Kulber DA, Choi A, et al. Keloid- derived, plasma/fibrin-based skin equivalents generate de novo dermal and epidermal pathology of keloid fibrosis in a mouse model. Wound Repair Regen. 2015; 24: 302–16.
- Yagi Y, Muroga E, Naitoh M, Isogai Z, Matsui S, Ikehara S, et al. An Ex Vivo Model Employing Keloid-Derived Cell–Seeded Collagen Sponges for Therapy Development. J Invest Dermatol. 2013; 133: 386–93.
- Supp DM. Animal Models for Studies of Keloid Scarring. Adv Wound Care. 2019; 8: 77–89.
- Festing MFW, Lovell D, Sparrow S, May D, Connors TA. An athymic nude mutation in the rat. Nature. 1978; 274: 365–6.
- Hayward P, Linares H, Evans M, McCauley R, Robson M. A model of human keloid using the nude (athymic) rat. Surg Forum. 1991; 42: 187–95.
- Polo M, Kim YJ, Kucukcelebi A, Hayward PG, Ko F, Robson MC. Anin VivoModel of Human Proliferative Scar. J Surg Res. 1998; 74: 187–95.
- Wang X, Smith P, Pu LLQ, Kim YJ, Ko F, Robson MC. Exogenous Transforming Growth Factor β2 Modulates Collagen I and Collagen III Synthesis in Proliferative Scar Xenografts in Nude Rats. J Surg Res. 1999; 87: 194–200.
- Sparks HD, Sigaeva T, Tarraf S, Mandla S, Pope H, Hee O, et al. Biomechanics of Wound Healing in an Equine Limb Model: Effect of Location and Treatment with a Peptide- Modified Collagen-Chitosan Hydrogel. ACS Biomater Sci Eng. 2021; 7: 265–78.
- Theoret CL, Olutoye OO, Parnell LKS, Hicks J. Equine exuberant granulation tissue and human keloids: A comparative histopathologic study. Vet Surg. 2013; 42: 783–9.
- Stashak T, Theoret C, editors. Wound repair in the horse: problems and innovative solutions. In: Equine Wound Management. 2nd ed. Wiley-Blackwell; 2008; 47–68.
- Gordillo GM, Bernatchez SF, Diegelmann R, Di Pietro LA, Eriksson E, Hinz B, et al. Preclinical Models of Wound Healing: Is Man the Model? Proceedings of the Wound Healing Society Symposium. Adv Wound Care. 2013; 2: 1–4.









































































































































