Coexistence of Cutaneous Tuberculosis and Systemic Sarcoidosis: A Diagnostic Dilemma
- 1. Department of Dermatology, Tunis El Manar University, Tunisia
Abstract
Introduction: Sarcoidosis is a multisystem granulomatous disorder of unknown etiology, characterized by the presence of non-caseating epitheloid cell granulomas. Several immune aberrations may play a role in its pathogenesis and mycobacterial infection in the pathogenesis of sarcoidosis has been suggested. In both clinical and histopathological features, sarcoidosis is remarkably similar to tuberculosis, very rarely, they may coexist.
Case report: A 52-year -old woman with dyslipidemia was referred to our hospital in March 2017 following a 3-month history of skin lesions. Her daughter is suffering from crohn’s disease. On physical examination, the patient had papules and purple scars on the face, chest, upper arms and hands; she was without fever during admission. No other physical abnormalities were observed. Laboratory investigations showed a white blood count 4600/mm3, C-reactive protein lower than 8mg/l, renal function indices were normal, a normal rate of liver enzyme. Tuberculosis skin test was 11 mm of in duration in diameter. Skin biopsy revealed granulomatous structures with central caseous necrosis. A computed tomogram of the thorax disclosed mediastinal lymphadenopathy and interstitial micronodular densities. Broncho-alveolar lavage (BAL) with trans-bronchial needle aspiration was performed; direct smear microscopy examination of the sample and sputum for the presence of acid-resistant bacilli by using Ziehl Neelson method was negative. However, the polymerase chain reaction (PCR) for M.Tuberculosis complex was not practiced. In the BAL, CD4+ lymphocytes and alveolar macrophages were predominantly. The ratio of CD4+ and CD8+ lymphocytes was over 2. All relative findings including patho-histological examination indicate lung sarcoidosis. On suspicion of human tuberculosis skin infection, standard anti-tuberculosis drug regimen was started (ISONIZID, RIFAMPICIN, PYRAZINAMIDE) entirely 9 months, ETHAMBUTOL was excluded because of dyschromatopsia. The patient did not receive any corticoids or antimalarial drugs in 2017. In total, the patient was treated with HRZ for 2 months followed by 7 months of HR. The skin lesions have disappeared progressively since the second month of treatment. In course of the last Month of TB therapy (February 2018), new nodes on face, nose, chest and upper arms, similar to those on the forehead has appeared.
Microbiological examination of the skin substrata in direct microscopy had not confirmed the presence of M.Tuberculosis. The biopsy of an erythematosquaous lesion of the left arm was taken which revealed granulomatous structures, consisting of epitheloid histiocytes and polynucleid grant cells, surrounded by small lymphocytes. No central necrosis or caseiting was seen. Energy to tuberculin was found. In March 2018, another computed tomogram of the thorax was performed which showed a regression of the size of mediastinal lymphadenopathy in comparison to earlier scan. Cardiac echography revealed discreet pericarditis which disappeared after 2 months. Nasal mucosa biopsy was practiced and showed no specific inflammatory tissue without granulomatous structures and absence of necrosis or malignancy. The patient was referred to the ophtalmologist to search an ocular location of sarcoidosis.
In May 2018, the patient received Colchicine therapy (1mg daily), and antimalarial drugs (Hydroxychloroquine 400mg daily) for lung and skin type of sarcoidosis and we noticed a remarkable improvement of skin lesions since the first month of treatment.
Keywords
Sarcoidosis; Tuberculosis; M.Tuberculosis
Citation
Khammouma F, Jaber K, Sayhi S, Charfi O, Rabhi F, et al. (2018) Coexistence of Cutaneous Tuberculosis and Systemic Sarcoidosis: A Diagnostic Dilemma. J Dermatolog Clin Res 6(2): 1119.
INTRODUCTION
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology, characterized by the presence of non-caseating epithelioid cell granulomas. Several immune aberrations may play a role in its pathogenesis and mycobacterial infection in the pathogenesis of sarcoidosis has been suggested. In both clinical and histopathological features, sarcoidosis is remarkably similar to tuberculosis, very rarely, they may coexist.
CASE PRESENTATION
A 52-year -old woman with dyslipidemia was referred to our hospital in March 2017 following a 3-month history of skin lesions. Her daughter is suffering from crohn’s disease. On physical examination, the patient had papules and purple scars on the face, chest, upper arms and hands (Figure 1);
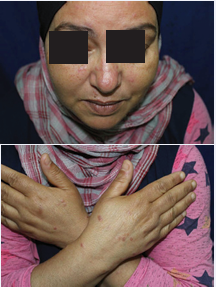
Figure 1 Papules and purple scars on the face and hands (Sarcoidosis).
she was without fever during admission. No other physical abnormalities were observed. Laboratory investigations showed a white blood count 4600/mm3, C-reactive protein lower than 8mg/l, renal function indices were normal, a normal rate of liver enzyme. Tuberculosis skin test was 11 mm of in duration in diameter. Skin biopsy revealed granulomatous structures with central caseiting necrosis. A computed tomogram of the thorax disclosed mediastinal lymphadenopathy (Figure 2)
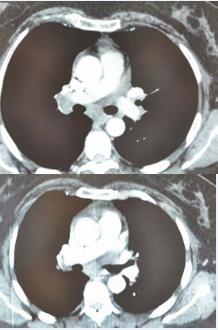
Figure 2 A comparison of regression of the size of mediastinal lymphadenopathy between 2017 and 2018.
and interstitial micro nodular densities. Broncho-alveolar lavage (BAL) with transbronchial needle aspiration was performed, direct smear microscopy examination of the sample and sputum for the presence of acid -resistant bacille by using Ziehl Neelson method was negative. However, the polymerase chain reaction (PCR) for M.Tuberculosis complex was not practiced. In the BAL, CD4+ lymphocytes and alveolar macrophages were predominantly. The ratio of CD4+ and CD8+ lymphocytes was over 2 (Figure 3).
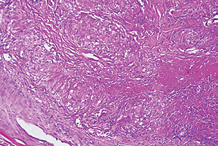
Figure 3 Granulomatous structures with central caseiting necrosis
All relative findings including patho-histological examination indicate lung sarcoidosis. On suspicion of human tuberculosis skin infection, standard anti-tuberculosis drug regimen was started (ISONIZID, RIFAMPICIN, PYRAZINAMIDE) entirely 9 months, ETHAMBUTOL was excluded because of dyschromatopsia. The patient did not receive any corticoids or antimalarial drugs in 2017. In total, the patient was treated with HRZ for 2 months followed by 7 months of HR. The skin lesions have disappeared progressively since the second month of treatment. In course of the last Month of TB therapy (February 2018), new nodes on face,nose, chest and upper arms, similar to those on the forehead has appeared.
Microbiological examination of the skin substrata in direct microscopy had not confirmed the presence of M.Tuberculosis. The biopsy of an erythematosquamous lesion of the left arm was taken which revealed granulomatous structures, consisting of epitheloid histiocytes and polynucleid grant cells, surrounded by small lymphocytes. No central necrosis or caseiting was seen (Figure 4).
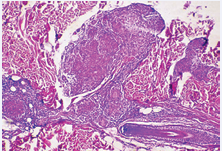
Figure 4 Granulomatous structures without central necrosis.
Energy to tuberculin was found. In March 2018, another computed tomogram of the thorax was performed which showed a regression of the size of mediastinal lymphadenopathy in comparison to earlier scan. Cardiac echography revealed discreet pericarditis which disappeared after 2 months. Nasal mucosa biopsy was practiced and showed no specific inflammatory tissue without granulomatous structures and absence of necrosis or malignancy. The patient was referred to the ophthalmologist to search an ocular location of sarcoidosis.
In May 2018, the patient received Colchicine therapy (1mg daily), and antimalarial drugs (Hydroxychloroquine 400mg daily) for lung and skin type of sarcoidosis and we noticed a remarkable improvement of skin lesions since the first month of treatment.
DISCUSSION
We report a rare case of 52-year-old female from Tunisia with skin lesions, mediastinal lymphadenopathy and interstitial micro nodular densities. The symptomatology was considered initially as skin Tuberculosis associated with pulmonary sarcoidosis and the patient received standard anti -tuberculosis drug regimen , the lesions had disappeared without corticosteroids or antimalarial drugs. One year later, she developed the same scars with a regression of the size of mediastinal lymphadenopathy. Many arguments are supporting tuberculosis infection; Tuberculosis infection is endemic in Tunisia, tuberculosis skin test was 11 mm of in duration in diameter with a caseating necrosis initially and the regression of the lesions after receiving HRZ. All relative findings including patho-histological examination support a tuberculosis skin infection associated with pulmonary sarcoidosis. But, the skin lesions are very similar to those seen in skin sarcoidosis and there are case reports of improvement of cutaneous sarcoidosis lesions with antibiotic therapy. Is it an association between skin tuberculosis and a systemic sarcoidosis since the beginning of the desease or a systemic sarcoidosis following a skin tuberculosis infection. It will still be a diagnostic dilemma.
Sarcoidosis is a common multisystem granulomatous disease that frequently involves the lungs and can result in pulmonary fibrosis [1]. Noncaseating epitheloid cell granulomas characterizing sarcoidosis may affect most organs, including the skin. Skin lesions may be the only manifestations, or just one of several other organ involvements. Cutaneous lesions are present in ~25% of sarcoidosis patients [2]. The skin lesions could have specific aspects (papules, plaques, nodules, alopecia or purple scars) or can be more or less non specific (erythema nodosum) [3]. Since sarcoidosis was first described, there has always been a belief that the disease is in some way related totuberculosis (TB) [4]. Sarcoidosis is known to follow or even coexist with TB [5], and TB following sarcoidosis is a fairly common event due to the immunosuppressive effects of glucocorticoids [6]. Thus, it is no surprise that a physician is likely to encounter situations wherein sarcoidosis may precede, follow or present concurrently with TB. Sarcoidosis is a granulomatous disease, which is likely a result of continued presentation of a poorly degradable antigen. Mycobacterium tuberculosis has been a very strong contender for this antigen. Besides the molecular studies demonstrating mycobacterial deoxyribonucleic acid (DNA) in the sarcoid tissue [7]. Wong et al reported a case of sarcoidosis in a patient previously treated for culture-proven TB and showed that one of the mechanisms of developing sarcoidosis is a continued reaction of M tuberculosis. It is known that mycobacterial infection leading to a positive skin test but with no viable organisms may still have positive PCR reactions that would imply that the organism has been effectively killed but its genetic material remains dormant in the lung. Mycobacteria can cause a granulomatous reaction and may cause a reaction indistinguishable from sarcoidosis [8]. Many authors hypothesize that prolonged exposure to mycobacterial proteins might stimulate host immune system to hypersensitivity reactions leading to non-caseating granuloma formation. Numerous epitopes of mycobacterial peptides (e.g., katG, ESAT6, CFP-10, hsp) have been found to serve as targets of adoptive immune response eliciting Th1 immunophenotype [9,10]. Various genetic factors, probably linked with HLA class I and II alleles, can further influence immunological response, leading to sarcoidosis or TB [11,12]. Strong immune reactions result in protection against progression of infection. This hypothesis seems to have epidemiological confirmation, as TB distribution worldwide is quite opposite to that of sarcoidosis. Furthermore, several studies using immune assays and the isolation of antigens [e.g. Mycobacterial catalase-peroxidase antigen, mKatG] have lead to a robust step in associating sarcoidosis and mycobacteria.
mKatG may lead to a T-cell response which leads to formation of granulomas and might therefore be a pathogenic antigen. These studies may indicate that the presence of mycobacterial antigens is enough to elicit immune reaction in a susceptible host. This might explain why only few case studies have been reported where patients develop sarcoidosis after culture positive tuberculosis and that the incidence of sarcoidosis is higher in countries with high burden of latent tuberculosis. This fact also highlights the limitations of serological and molecular studies to discriminate between the two conditions [13].
Current treatments of sarcoidosis focus on limiting the inflammatory response, with corticosteroids serving as the mainstay of therapy [14]. Among non-steroid based treatments, only infliximab has been shown in a randomized, placebocontrolled study to improve lung function [15]. There are case reports of improvement of cutaneous sarcoidosis lesions with antibiotic therapy, such as tetracyclines [16,17]. Also present within sarcoidosis granulomas are genes encoding mycobacterial products that can be targeted by antimycobacterial therapy such as rpoB[rifampin] [18] andDNA gyrase A(levofloxacin) [19]. There are anecdotal reports of treating patients with anti-tubercular drugs and steroids in patients with TB where sarcoidosis was suspected as well [20]. More recently, Drake and colleagues published the data from phase 2 of CLEAR trial showing excellent clinical response in patients with pulmonary and skin sarcoidosis after treatment with broad-spectrum anti-mycobacterial therapy [14]. Although initial results are fascinating, those studies raise many questions as well.









































































































































