Compositions for Prevention or Prophylactic Treatment of Poison Ivy Dermatitis
- 1. ElSohly Laboratories, Inc., 5-Industrial Park Drive, USA
- 2. National Center for Natural Products Research, University of Mississippi, USA
- 3. Department of Pharmaceutics and Drug Delivery, University of Mississippi, USA
- 4. Department of Pharmaceutics and Drug Delivery, University of Mississippi, USA
Abstract
Allergic contact dermatitis (ACD) to poison ivy and related species of the family Anacardiacea is a major cause of occupational dermatitis in the United States. Although the condition is not life threatening, it imposes significant risks to individuals with outdoor occupations and those engaged in outdoor activities. This results in significant healthcare costs and loss of productivity. Efforts to prevent poison ivy ACD have been pursued for years, and trials to produce desensitization through administration of small doses of the allergens (urushiols) have been largely unsuccessful and therefore, abandoned. Topical blocking agents applied to the skin to protect it from contact with the urushiols are of limited value and require application before exposure to the allergen. Development of an effective prophylactic treatment to poison ivy ACD is, therefore, of high value. This manuscript reports on the development of water soluble derivatives of the saturated congener on the poison oak urushiol (3-n-heptadecylcatechol, HDC) for prophylactic treatment of poison ivy ACD. Three compounds were prepared, namely the HDC-phenylalaninate (1), the HDC-hemiglutarate (2), and the HDC-4-(4-aminophenyl)-butyrate (3). These derivatives were shown to produce tolerance against sensitization to poison ivy urushiol in guinea pigs (the animal model for allergic contact dermatitis) when administered prior to sensitization of animals (i.e. naïve guinea pigs). Furthermore, upon IM administration of compound 3 to animals with established sensitivity to poison ivy, the product resulted in almost complete desensitization to urushiol. This suggests that these compounds are good candidates for development as prophylactic treatment for poison ivy ACD.
Keywords
Prophylactic; HDC-Phenylalaninate; HDCHemiglutarate; HDC-4-(4-Aminophenyl)-Butyrate; Urushiol
Citation
ElSohly MA, Gul W, Abdel-Bakky MS, Manly SP, Ashfaq MK (2018) Compositions for Prevention/Prophylactic Treatment of Poison Ivy Dermatitis. J Dermatolog Clin Res 6(1): 1115,
ABBREVIATIONS
ACD: Allergic Contact Dermatitis; ACS: American Chemical Society; EtOH: Ethanol; HDC: 3-n-Heptadecylcatechol; HDC-APB: HDC-4-(4-aminophenyl)-butyrate; HDC-HG: HDC-Hemiglutarate; IM: Intramuscular
INTRODUCTION
Contact dermatitis to poison ivy (Toxicodendron radicans), poison oak (T. diversilobum), and poison sumac (T. vernix) affects 10-50 million Americans every year [1] and is the primary cause of occupational dermatitis in the United States [2]. The prevalence of poison ivy and poison oak sensitivity in the general adult population ranges from 50% to 70% [3,4]. Peak frequency for sensitization occurs between ages 8-14 [5]. Genetic susceptibility to urushiol sensitivity suggests that 80% of children who are born to two urushiol sensitive parents will become sensitive [6]. Outdoor activities as well as outdoor occupations such as fire fighting, forestry and agriculture are at high risk for incurring significant medical expenses and worker’s disability. Each fire season, approximately one third of forestry workers in California, Oregon and Washington are disabled by poison oak dermatitis [7]. This disorder is very well known to most emergency and primary care physicians and dermatologists [8].
Other genera of the plant family Anacardiaceae with dermatogenic constituents include Anacardium (cashew nuts), Semicarpus (India ink tree), Metopium (poison wood), and Mangifera (mango). The allergenic components in most of these plants are 3-n-alk-(en)-yl catechols with C-15 or C-17 side chains and different degrees of unsaturation (0-3 olefinic bonds) [9- 12]. Urushiol is typical of such allergenic components present in poison ivy, poison oak, and the Asian lacquer tree [13]. It has a catechol ring substituted with a C15 or C17 hydrocarbon chain at the 3 or 4 positions, either saturated or having one, two or three unsaturated bonds [14]. Both the catecholic ring and the aliphatic chain are proven to play important roles in allergenicity of urushiols [15-17]. Contact of these catechols with the skin of susceptible individual’s results in sensitization to all urushiols of the plant family Anacardiaceae [18]. Once sensitivity is developed, it is difficult, if not impossible, to eliminate.
Current treatment for contact dermatitis is primarily symptomatic. For patients with severe cases, a tapering dose of oral corticosteroids such as prednisone may be used. Prednisone is a corticosteroid hormone (glucocorticoid) which suppresses the immune system’s response to various diseases to reduce symptoms such as swelling and allergic-type reactions. However, available “dosepacks” of corticosteroids are of little use since they deliver small doses of corticosteroid for too short a period of time and often result in a rebound reaction [19].
There have been multiple desensitization regimens since the 1950’s using extracts of poison ivy/oak; none are reliably effective [20,21]. The techniques consisted of ingestion or parenteral injection of various formulations of urushiol. Although some reports described success, the level of desensitization was variable and not durable. In addition, the regimens produced inflammation of mucous membrane, cutaneous, and systemic side effects, hence, this approach has been largely abandoned.
The precutaneous absorption of urushiol is similar to that of other lipophilic substances. These molecules preferentially enter the skin through the intercellular lipids of the stratum corneum. Any substance that blocks the contact of urushiol with the stratum corneum and prevents its entry would likely offer some protection. Many commercial products have been developed and tested for their effectiveness in preventing urushiol dermatitis, and these experiments have been published [2,22-28]. Presently, only a few substances offer some realistic benefits. Furthermore, the consumer of these products is assumed to have prior knowledge that the person will be exposed to poison ivy so topical application could be applied to the skin beforehand. Such is not the case with most situations when poison ivy is encountered. Therefore, the development of a prophylactic treatment for poison ivy dermatitis is a more realistic and a more effective strategy, if it could be achieved.
Hyposensitization by administration of plant extracts is not regularly obtained. It requires large doses and months or years to be produced, and sensitivity is rapidly regained upon cessation of such treatment [18, 20]. The benefits and safety of the use of Rhus extracts (containing the active allergenic ingredient urushiols) for these purposes have been topics of dispute since they were first administered in 1917. Several reviews pertaining to the clinical use of Rhus extracts and allergens have been written [22,29,30].
The reason for the lack of activity of administered urushiols in the free form might be due to the high reactivity of the catechol moiety of the urushiols with plasma proteins. Once absorbed, the urushiols bind irreversibly with the proteins and become “deactivated”. From our earlier studies, we concluded that it might be necessary for the urushiols to bind to cell membranes to be effective in the production of tolerance or the prophylactic treatment of poison ivy dermatitis. Thus, a conjugate of poison ivy urushiol bound to cell membranes was prepared by spiking the urushiol solution into a suspension of blood cell membranes from lyzed and washed blood cells and then re-injected the suspension into donor animals [18]. We have shown in that study that tolerance was produced by the administration of 3-n-pentadecylcatechol (the saturated congener of poison ivy urushiol) coupled to red blood cell membranes in guinea pigs. The treated group was tolerant to 3-n-pentadecylcatechol for the 20 weeks of the study. Having succeeded in that approach, we theorized that administration of an urushiol ester might be more effective in that some of the ester could hydrolyze at the surface of the blood cells resulting in free urushiol which could bind to the membrane. Tolerance to poison ivy urushiol in the guinea pig model was accomplished by IV injection of the diacetate esters of poison ivy and oak urushiols in na?ve guinea pigs, and complete desensitization or hyposensitization was accomplished in sensitized animals by the same treatment [31]. The efficacy of oral administration of poison ivy and poison oak urushiols was compared with the use of the respective esterified derivatives for desensitizing sensitive guinea pigs [32]. The esterified derivatives produced a greater degree of hyposensitization than was produced by the free urushiols. However, the effect of the orally administered preparation was not substantial. We have concluded that for the urushiol esters to be most effective, parentral administration is necessary. We, therefore, conducted a study to evaluate the potential for a single-dose regimen to be effective for hyposensitization to poison ivy urushiol dermatitis [33]. Hyposensitization was accomplished in a single intramuscular dose of 20 mg.
his publication deals with the development of a group of polar derivatives of urushiol (or its saturated congener) (Figure 1)
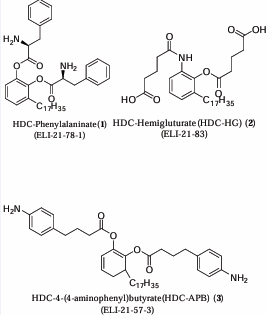
Figure 1 Structures of HDC-Phenylalaninate (ELI-27-78-1) (1), HDC-Hemigluturate (HDC-HG) (ELI-21-83) (2), and HDC-4-(4 aminophenyl) butyrate (HDC-APB) (ELI-21-57-3) (3).
that would be administered as salts of these derivatives in an aqueous solution for a successful product for the prevention of poison ivy/poison oak contact dermatitis. Furthermore, this product could be used to provide tolerance to urushiols sensitization when administered to naïve individuals (i.e. prevents subjects from ever being sensitized to urushiols upon first exposure), or to provide desensitization to urushiol (i.e. blocks the reaction of a sensitive individual upon exposure to urushiol) when administered to already sensitized individuals.
MATERIALS AND METHODS
The novel agents (Figure 1) were synthesized, purified, and characterized by spectral analysis techniques following the procedures outlined in US Patent #8,486,998 B2 [34]. A guinea pig contact dermatitis model was used for in vivo efficacy studies.
Chemicals
All chemicals and solvents were of ACS grade (Sigma-Aldrich, Milwaukee, WI, USA).
Animals
Hartley strains of guinea pigs (n=40) were obtained from Harlan (Indianapolis, IN 46229). The animals were divided into 5 groups (n=8/group) and treated as described below. These animals were kept under controlled environment with 12 hour day and night cycle and provided feed and water ad libitum. The animal study protocol was approved by the IACUC of the University of Mississippi (IACUC protocol# 06-007).
Study design (See diagram in Figure 2)
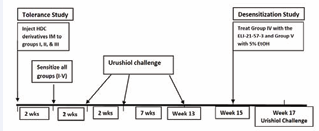
Figure 2 Schematic study design showing treatments given at various times during the experiment.
A. Production of tolerance to poison ivy/oak urushiol in naïve guinea pigs using water soluble derivative of 3-nheptadecylcadetol:
The 5 groups of animals were treated as follows:
Group I. Animals in this group were given 300 μL of the compound ELI-21-57-3 (HDC-APB) (3) via the intramuscular (IM) route, in each hind leg, containing the equivalent of 3 mg HDC/leg. Two weeks later, these animals were sensitized with urushiol (100 μL acetone containing 1.0 mg of urushiol) on the skin of the dorsal side of the neck region. Two weeks later, the animals were challenged with urushiol (15 μL volume acetone containing 3.0 μg, or 4.5 μg or 6.0 μg) on the abdominal skin. The vehicle was 15μL of acetone (see Figure 3).
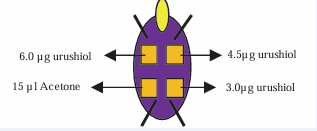
Figure 3 Sites on the abdominal surface of skin for application of urushiol challenge doses dissolved in 15 μL acetone vehicle.
The animals were challenged three times after sensitization. (viz; first challenge (Test #1) at two weeks post sensitization, second challenge (Test #2) conducted at four weeks post sensitization and third challenge (Test #3) was conducted twelve weeks post sensitization).
Group II. Animals were given 300 μL of the compound ELI21-78-1 (HDC-phenylalaninate) (1) via the IM route in each hind leg, containing the equivalent of 3 mg HDC/leg. This was followed by sensitization and then challenging following the same procedure as for Group I.
Group III. Animals were given 300 μL of the compound ELI-21-83 (HDC-HG) (2) via the IM rout in each hind leg, containing the equivalent of 3 mg HDC/leg. This was followed by sensitization and then challenging following the same procedure as for Group I.
Group IV. Animals were given 300 μL of vehicle (5% Ethanol) via the IM route in each of the hind legs. This was followed, two weeks later, by sensitization and abdominal skin test as described for Group I.
Group V. Animals in this group were given PBS (300 μL in each of the hind legs) via the IM route. Two weeks later, the animals were sensitized and then challenged following the same protocol described for Group I.
B. Desensitization to poison ivy/oak urishiol of already sensitive guinea pigs using water soluble derivatives of 3-n-heptadecylcatechol: The control animal groups, Groups IV and V, vehicle and phosphate buffer control groups respectively, from the tolerance study (untreated with the derivatives), with established sensitivity (see Figures 4 and 5)
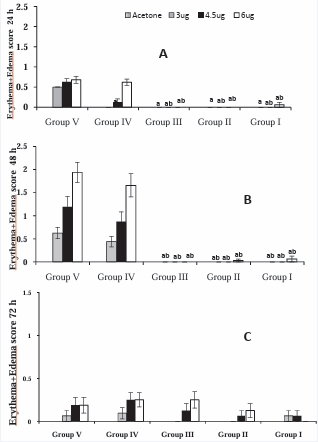
Figure 4 (A-C): Test #1 -Guinea pigs in different groups were injected IM with different treatments: Group I: HDC-APB (ELI-21-57-3), Group II: HDC-phenylalaninate (ELI-21-78-1), Group III: HDC-HG (ELI-21-83), Group IV: 5% EtOH, and Group V: PBS. Two weeks post treatment; guinea pigs were sensitized with 1.0 mg urushiol on neck area. Two weeks after sensitization, the guinea pigs were challenged with acetone, or acetone containing 3.0, 4.5, or 6.0 µg urushiol at each of the four abdominal sites. Erythema+edema scores for the animals were tabulated. Data represents group means ± SEM. aSignificantly different from Group V (PBS) at the same challenge dose bSignificantly different from group IV (5%EtOH) at the same challenge dose.
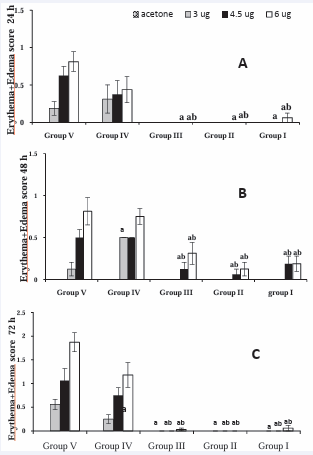
Figure 5 (A-C): Test #2 - Guinea pigs in different groups were injected IM with different treatments: Group I: ELI-21-57-3, Group II: ELI 21-78-1, Group III: ELI-21-83, Group IV: 5% EtOH, and Group V: PSB. Two weeks post treatment; guinea pigs were sensitized with 1.0 mg urushiol on neck area. Four weeks after sensitization, the guinea pigs were challenged with acetone, or acetone containing 3.0, 4.5, or 6.0 µg urushiol at each of the four abdominal sites. Erythema+edema scores for the animals were tabulated. Data represents group means ± SEM. asignificantly different from Group V (PBS) at the same challenge dose. bsignificantly different from group IV (5% EtOH) at the same challenge dose.
were used in this study to determine if IM administration of one of the water soluble derivatives would desensitize already sensitive animals.
Animals of Group IV were given 600 μL of vehicle divided into two doses of 300 μL in each hind leg. Animals of Group V were injected with 600 μL (300 μL in each hind leg) of ELI-21-57-3 (3) solution containing the equivalent to 10 mg/mL of HDC (6 mg/animal dose). After a rest period of approximately 2 weeks, animals in both groups were challenged by topical application of 3 doses of urushiol (3.0 μg, 4.5 μg, and 6.0 μg each dissolved in 15 μL of acetone). At the 24, 48, and 72 hours post topical challenge, skin lesions were observed and scored.
The severity of erythema and edema was observed and scored according to the Driaze scoring system [35] as shown in Table 1. The scores were recorded at the 24, 48, and 72 hrs post urushiol skin application.
Statistical analysis
Erythema-edema scores for different groups were analyzed by one-way ANOVA followed by the Tukeys test for multiple comparisons using the Graph Pad prism 5.0 software (La Jolla, CA). A P-value of less than 0.05 was considered to show a significant difference in the treated and untreated groups.
RESULTS AND DISCUSSION
In previous investigations [34,36-37], we have shown that tolerance, as well as desensitization, are accomplished by the injection of the acetate esters of poison ivy urushiol. In continuation of this work, and to develop better drugs for the prophylactic treatment of contact dermatitis caused by poison ivy, poison oak, and related species of the family Anacardiaceae, we have prepared water soluble salts of amino acid esters and dicarboxylic acid esters of the saturated congener of poison oak and tested their ability to convey tolerance and desensitization,
using the guinea pig model of contact dermatitis. Three derivatives of n-heptadecylcatechol (HDC) were prepared, namely the phenylalaninate (1), the hemiglutarate (2) esters, and the 4-(4-aminophenyl)-butyrate (3) (Figure 1). These compounds were formulated in aqueous solutions containing 5% ethanol at the equivalent of 6 mg/mL of HDC for each derivative.
Two studies were carried out using 5 groups of animals (8 guinea pigs/group).
Study #1: tolerance study
A) Production of tolerance to poison ivy/oak urushiol in naïve guinea pigs using water soluble derivatives of 3-n-heptadecylcatedol: Groups I-III were injected with the three derivatives (one derivative/group) at a total dose of 6 mg/animal (0.3 mL of the derivative solution per hind leg of the animal for a total dose of 0.6 mL). Group IV was injected with the vehicle and group V with phosphate buffer as controls. Two weeks after dosing, all animals were skin sensitized with poison ivy urushiol (1 mg urushiol applied to the shaved dorsal side of the neck). Subsequently, the animals were checked for sensitization by the application of three doses of urushiol (3, 4.5, and 6 µg each in 15 μL of acetone) to the shaved abdominal skin. A solvent control was also used. Three tests for induction of tolerance were carried out as follows:
Test #1: In the first test, animals were challenged two weeks after sensitization. Guinea pigs in groups IV and V (PBS or 5% ethanol treatment, respectively) showed varying degrees of erythema and edema with urushiol challenge doses of 3.0, 4.5, and 6.0 μg. In contrast, guinea pigs in group I (ELI-21-57-3) showed only a slight reaction at the 6.0 μg urushiol challenge. Animals in group II (ELI-21-78-1) and group III (ELI-21-83) showed no reaction at 3.0 and 4.5 μg urushiol challenges. In Group I, at the 6.0 μg urushiol challenge, a very slight reaction was observed.
Table 1: Draize scoring system used to score the severity of erythema and edema.
| Skin Lesion Observed | Score |
| No Erythema | 0 |
| Very Slight Erythema (barely perceptible) | 1 |
| Well Defined Erythema | 2 |
| Moderate to Severe Erythema | 3 |
| Severe Erythema (beet red) Eschar Formation (deep injury) | 4 |
| No Edema | 0 |
| Very Slight Edema (barely perceptible) | 1 |
| Well Defined Edema (edges of area defined by definite raising) | 2 |
| Moderate Edema (area raised approximately 1 mm) | 3 |
| Severe Edema (raised more than 1 mm and extending beyond area of exposure) | 4 |
| Maximum Summed Erythema and Edema Scores = 8 | |
A statistically significant (P < 0.05) reduction in skin reaction scores was observed in the treated animal groups I, II, and III, ascompared with those of the control groups IV and V (Figure 4A). At 24 hours, the skin reaction scored in the vehicle treated group was lower than that of PBS treated at the challenged score of 3.0 and 4.5 μg urushiol. However, there was no difference in the scores of these two groups at the challenge dose of 6.0 μg. As expected, the challenge (acetone vehicle) showed no reaction (Figure 4A).
At 48 hours, the skin reaction to the urushiol challenge in groups IV and V showed the dose response phenomenon: the reaction score to the urushiol dose 3.0 μg being the lowest, followed by 4.5 μg, and 6.0 μg being the highest. However, in groups I, II, and III, no or minimal reaction was observed with 3.0, 4.5, or 6.0 μg urushiol challenge. No erythema or edema was observed in the three prophylactic treated groups. In the PBS and vehicle treated animals, the skin reaction scores were higher with the increasing concentration of urushiol challenge (Figure 4B). The treated groups I, II, and III did not show any reaction to the urushiol challenge dose of 3.0 or 4.5 μg. A slight reaction was observed at 6.0 μg urushiol dosage in groups I and II. The reaction scores of the treated groups were significantly different (P < 0.05) from that of the control groups IV and V (Figure 4B).
The observations at 72 hours remained similar to those observed at 48 hours. No to minimal skin reaction was observed in groups I, II, and III. The control groups showed skin reaction scores for groups IV and V to be comparable but tended to be slightly higher in the PBS group (Figure 4C). Statistically, the skin reaction scores of the treated groups (I, II, and III) were significantly different (P < 0.05) from the vehicle or PBS treated groups (IV and V) at the respective doses of urushiol challenge. Comparison of lesion scores of the PBS and vehicle groups at different time points indicates that maximum erythema and edema were observed at 48 hours. At 72 hrs, the lesions tended to subside, as compared to those at 48 hours.
In all groups, no skin reaction was observed at the site of acetone (without urushiol) application on the skin, indicating that the reaction was exclusively against urushiol.
Test #2: In the second test, guinea pigs were challenged four weeks after sensitization (two weeks after the first challenge). At 24 hours, the skin reaction scores of the animals in groups I, II, and III, prophylactically treated with ELI-21-57-3, ELI-21-78-1, and ELI-21-83 respectively, were significantly lower (P < 0.05) than those in group IV (vehicle, 5% EtOH) or group V (PBS) at the respective doses of urushiol challenge (Figure 5A).
At 48 hours, a very slight reaction was observed in the treated groups I, II, and III to 4.5 and 6.0 μg doses of urushiol. No reaction was seen at the 3.0 μg dose of urushiol in these groups. These scores were significantly (P < 0.05) lower than those of vehicle (group IV) and PBS (group V) (Figure 5B).
At 72 hours, the reaction tended to regress. The skin reaction scores in the treated groups I, II, and III were comparably lower than those in the control groups IV and V. However, they are not statistically significant (Figure 5C).
Test #3: The third test was conducted approximately seven weeks after the second test. At 24 hours, Groups I, II, and III did not show erythema or edema at the site of urushiol challenge at any of the three doses used. Animals in groups IV (vehicle) and V (PBS) showed a dose response to the urushiol challenge at doses of 3.0, 4.5, and 6.0 μg. The skin reaction scores of the treated groups I, II, and III were significantly lower (P < 0.05) than the control groups IV and V (Figure 6A).
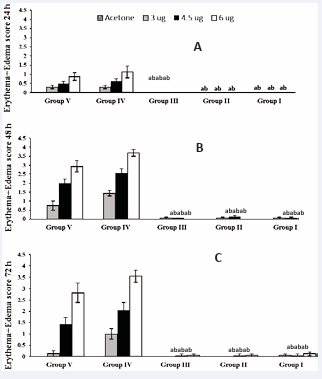
Figure 6 (A-C): Test #3 - Guinea pigs in different groups were injected IM with different treatments: Group I: ELI-21-57-3, Group II: ELI 21-78-1, Group III: ELI-21-83, Group IV: 5% EtOH, and Group V: PBS. Two weeks post treatment; guinea pigs were sensitized with 1.0 mg urushiol on neck area. Eleven weeks after sensitization, the guinea pigs were challenged with acetone, or acetone containing 3.0, 4.5,or 6.0 µg urushiol at each of the four abdominal sites. Erythema+edema scores for the animals were tabulated. Data represents group means ± SEM. asignificantly different from Group V (PBS) at the same challenge dose. bsignificantly different from group IV (5%EtOH) at the same challenge dose.
At 48 hours, the skin reaction scores to the three doses of the urushiol challenge in Groups I, II, and III remained significantly lower compared to those of the control groups IV and V (Figure 6B).
At 72 hours, the skin reaction was similar to that seen at 48 hours (Figure 6C compared with 6B). The skin reactions to the three doses of urushiol challenge remained significantly lower compared to those of the control groups IV and V (Figure 7).
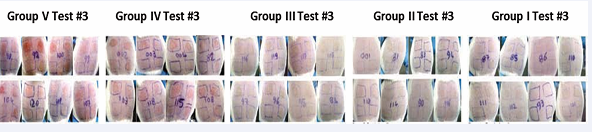
Figure 7 Skin reactions of groups I through V at 24 hours post application of poison ivy urushiol in Test #3.Skin reaction of guinea pigs at 24 hours post challenge of test #3. Guinea pigs in different groups were treated IM as follows: Group I: ELI 21-57-3; Group II: ELI 21-78-1; Group III: ELI 21-83; Group IV: 5% EtOH, and Group V: PBS. Two weeks post IM treatment; guinea pigs were sensitized with 1.0 mg urushiol on neck area. Eleven weeks after sensitization, the guinea pigs were challenged with acetone or acetone containing 3.0, 4.5, or 6.0 µg urushiol at each of the four abdominal sites.
A visible difference was observed in erythema at the sites of topical application of urushiol in groups IV and V, as compared to groups I, II, and III.
Study #2: Desensitization study
B) Desensitization to poison ivy/oak urushiol of already sensitive guinea pigs using the water soluble prodrug of 3-n-heptadecylcatechol HDC-APB (ELI-21-57-3)
The control animal groups IV and V, (5% EtOH and PBS control groups respectively), from the tolerance study, with established sensitivity (see Figure 6, groups IV and V) were used in this study to determine if IM administration of one of the water-soluble derivatives prepared would desensitize already sensitive animals.
Animals of group IV were injected 600 μL (300 μL in each hind leg) of ELI-21-57-3 (HDC-4-(4-aminophenyl)-butyrate ester) solution at 57 mg/kg. Animals in group V were given 600 μL of vehicle (5% ethanol in water) divided in two doses of 300 μL in each hind leg. After a rest period of approximately 2 weeks, animals in both groups were challenged by topical application of 3 doses of urushiol (3 μg, 4.5 μg, and 6.0 μg each dissolved in 15 μL of acetone). At the 24, 48, and 72 hours post topical challenges, skin lesions were observed and graded as described earlier.
The photomicrograph in Figure 8 shows that at 24 hours, no skin reaction was observed in the animals of Group IV treated with ELI-21-57-3. In Group V (5% EtOH vehicle control), no skin reaction was observed with 3.0 μg urushiol challenge. However, at the 4.5 and 6.0 μg urushiol challenges, skin reactions were observed.
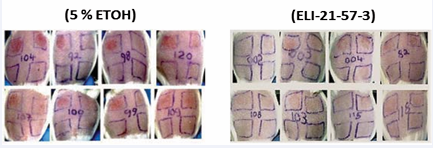
Figure 8 Skin reaction to poison ivy urushiol challenge at 72 hours. Previously sensitized guinea pigs were treated IM with ELI-21-57-3 (HDC-APB) or 5% EtOH.
At the 48 hours post topical challenge with urushiol, the animals in group IV (treated with ELI-21-57-3) showed no reaction with 3.0 μg, and a slight reaction with the 4.5 and 6.0 μg urushiol challenges. In contrast, the animals in group V (5% EtOH) showed a dose response reaction to 3.0, 4.5, and 6.0 μg of urushiol challenge. The skin reaction score of the treated group (group IV) were significantly lower than those of group V (5% EtOH treated group V).
Figure 9 shows the severity of skin reaction score in groups IV and V.
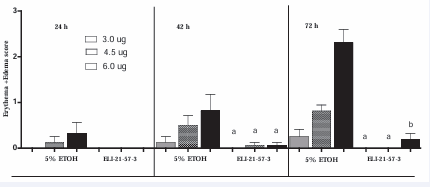
Figure 9 Guinea pigs were sensitized first with 1.0 mg urushiol in neck area. Two weeks after Test #3, the guinea pigs of Group IV (previously treated with EtOH) animals were injected IM with ELI-21-57-3, while group V guinea pigs (previously treated with PBS) were now given IM injection of 5% EtOH. Two weeks post treatment, both groups were challenged with 100 µL of acetone containing 3.0, 4.5, or 6.0 µg urushiol at each of the four abdominal sites. Erythema and edema score at each site was recorded at 24, 48 and 72 hours post urushiol challenge. Data represents mean score ± SEM, for group IV (ELI-21-57-3 treated) or group V (5 %EtOH treated) in the graph. a(p<0.05) compared to PBS treatment at 48 and 72 hours at the same dose of urushiol challenge. b(p<0.001)compared to PBS treatment at 72 hours at the same dose of urushiol challenge. *Acetone was not administered to any of the guinea pigs, due to the lack of reactivity seen in the tolerance study.
At the 72 hours post topical challenge, the ELI-21-57- 3 treated animals showed subdued reaction at the highest does (6.0 μg) of urushiol challenge. At lower doses, no reaction was visible. In contrast, 5% EtOH animals showed reaction to all three doses of urushiol. A pronounced reaction to urushiol challenge was observed at the highest dose (6.0 μg). Comparison of skin reaction scores of group IV animals were significantly (p<0.05) lower than those of the 5% EtOH treated group.
This indicates that an IM injection of the test compound (ELI21-57-3, HDC-APB) effectively desensitized previously reactive animals.
This study is composed of two portions, one related to the induction of tolerance to naïve animals towards sensitization to poison ivy urushiol and the other relating to the development of desensitization to poison ivy in animals already sensitive to urushiol. In the tolerance study, the skin lesion scores in Groups I, II, and III in all the three tests were negligible compared to the control Groups IV and V (untreated). This indicates that an intramuscular injection of any of the three test compounds protected the animals against sensitization to poison ivy dermatitis. All three compounds were equally effective, as no remarkable difference was observed in the skin lesion scores of these three groups.
The skin reaction of Groups IV and V in Test #1 and Test #2 were not as severe compared to those observed in Test #3. It is possible that the high sensitizing dose of urushiol (1.0mg) on the neck may have caused a state of “anergy”. This condition is observed in patients of tuberculosis (TB), who are burdened with huge amount of TB antigen, but show no reaction to the intradermal TB test (false negative). However, a rest period of 11 weeks between sensitization and Test #3 perhaps reversed the anergic state to a normal reactive state. Thus, in Test #3, animals in the Groups IV and V (untreated) exhibited strong skin reactions to urushiol challenge, while animals in Group I, II, and II were protected due to the prophylactic treatment. Therefore, tolerance was produced, which protected the guinea pigs against sensitization to poison ivy urushiol, by the administration of the test compounds to the animals prior to attempted sensitization.
f inducing desensitization to poison ivy urushiol, groups IV and V, which are already sensitive to urushiol were used in a desensitization experiment. Group IV was treated with one of A B C ababab ababab ababab ababab ababab ababab ababab Figure 6 (A-C): Test #3 - Guinea pigs in different groups were injected IM with different treatments: Group I: ELI-21-57-3, Group II: ELI21-78-1, Group III: ELI-21-83, Group IV: 5% EtOH, and Group V: PBS. Two weeks post treatment; guinea pigs were sensitized with 1.0 mg urushiol on neck area. Eleven weeks after sensitization, the guinea pigs were challenged with acetone, or acetone containing 3.0, 4.5,or 6.0 µg urushiol at each of the four abdominal sites. Erythema+edema scores for the animals were tabulated. Data represents group means ± SEM. a significantly different from Group V (PBS) at the same challenge dose. b significantly different from group IV (5%EtOH) at the same challenge dose. the test compounds (ELI-21-57-3, HDC-APB) while Group V was administered the vehicle. Two weeks after treatment, the animals were skin tested.
Figure 9 shows that, at 24 hours, no skin lesions were observed in animals that were given ELI-21-57-3 (HDC-APB). In the vehicle group, no skin reaction was observed with 3.0 μg urushiol. Very slight erythema was observed at 4.5 µg and 6.0 µg urushiol. At the 48 post hrs topical challenge, there was dose response of skin reaction to with 3.0, 4.5, 6.0 µg urushiol in the vehicle group, whereas the ELI-21-57-3 (HDC-APB) group showed no or very slight erythema. At the 72 hours post urushiol challenge, in the vehicle group (5% EtOH), the skin reactions to 3.0, 4.5, and 6.0 µg urushiol was more pronounced as to compare to the 24 and 48 hr. However, in the group treated with ELI-21-57-3, there was no skin reaction in response to the urushiol challenge at 3.0 and 4.5 µg but a slight reaction at 6.0 µg. Significant reduction in skin reaction to the urushiol challenge was observed at 48 and 72 hrs in the treated (ELI-21-57-3) group (see Figure 9). This shows that treatment of already sensitive animals with one of the test compounds (ELI-21-57-3, HDC-APB) resulted in desensitization of the treated animals to poison ivy urushiol.
Results from the desensitizing study also showed a visible reduction in erythema and edema at the site of topical application of urushiol in the group treated with HDC-APB. This shows that treatment of already sensitive animals with one of the test compounds (HDC-APB) resulted in desensitization of the treated animals to poison ivy urushiol. These results complement our previous work where guinea pigs were desensitized with urushiol acetate via the intravenous (IV) route and multiple or single dose of treatments via the oral route [32,36-37]. However,in this study, HDC-APB was administered as a single dose via the intramuscular (IM) route.
CONCLUSION
The tolerance induction portion of this study showed that an IM administration of any of the water soluble prodrugs (6 mg equivalent HDC/animal) induced tolerance to the test groups, whereby no (or very slight) skin reaction was observed in the treated groups as compared to the control (untreated) groups.
All compounds were equally efficacious.
Furthermore, treatment of already sensitive animals with one of the test compounds (HDC-APB) resulted in desensitization of the treated animals to poison ivy urushiol.
This shows that the test compounds are capable of producing tolerance when administered to naïve animals, and desensitization when administered to already sensitive animals, making them good candidate drugs for prophylactic treatment of poison ivy contact dermatitis.
ACKNOWLEDGEMENTS
This project was supported by NIH STTR grant funds from 1 R41-AR053395-01 and 1 R41-AR053395-01 and partially supported by Hapten Sciences, Inc.









































































































































