Effects of Bosentan Therapy on Circulating Endothelial Cells and Endothelial Progenitors and Capillaroscopic Patterns in Patients with Systemic Sclerosis
- 1. Department of Medicine, University of Verona, Italy
- 2. Department of Experimental Medicine, University of Genoa, Italy
Abstract
Background and objective: Several studies have recently demonstrated that circulating endothelial cells are increased in patients affected by Systemic Sclerosis (SSc) and have assessed a strong correlation between their number and vascular damage. Endothelial progenitor’s cells are strongly involved in vascular repair in response to vascular damage, both promoting vasculogenesis and activating angiogenesis. In SSc a defective vasculogenesis inhibits the vascular repair machinery. It is well known the effects of the dual endothelin-1 (ET-1) receptor antagonist, bosentan, on vasodilatation and the positive clinical effects on features such as Raynaud’s phenomenon, skin ulcers and pulmonary arterial hypertension.
We tested the hypothesis that in patients with SSc oral administration of bosentan may interfere with circulating endothelial cells and progenitor endothelial cells and with their effects on vascular damage.
Methods: We enrolled 20 patients affected by SSc (13 patients with and 7 patients without digital ulcers) and 10 sex and age matched healthy controls. Clinical features, laboratory tests and nailfold capillaroscopy were analyzed at baseline and after 3-6 and 12 months from the beginning of the treatment in the subgroup of patients and at the enrollment in the control group. Blood samples were collected from healthy controls at baselineand from all patients before and at different time after bosentan treatment were started. Circulating endothelial cells and endothelial progenitor’s cells were detected in the peripheral blood of SSc patients and controls by flow cytometry. Statistical analysis to assess any significant correlation between clinical features and endothelial cells was performed with the SPSS 21 statistical package.
Results: The number of both circulating endothelial cells and progenitors correlates with SSc diagnosis, with the presence of digital ulcers and with capillary density and disorganization of the capillary architecture shown bynaifold capillaroscopy. Circulating endothelial cells and progenitors number both increased after the beginning of bosentan therapy as well as improvement of skin ulcers and late improvement of capillaroscopic pattern. No correlation with other clinical features and endothelial cells count was found.
Conclusion: In SSc both endothelial cells and endothelial progenitors have a role as predictors of vascular damage, since they are strongly related to skin involvement, in particular with the presence of digital ulcers, and to capillary loss or disorganized structure demonstrated by naifoldcapilloroscopy. In this setting, treatment with dual ET-1 receptor antagonist bosentan due to an increase in both endothelial cells and progenitors and may modulate an effect on vascular repair.
Keywords
Systemic sclerosis; Endothelial cells; Endothelial cell progenitors; Nailfold videocapillaroscopy; Bosentan
Citation
Elisa T, Alessandro B, Ruggero B, Antonio P, Claudio L (2020) Effects of Bosentan Therapy on Circulating Endothelial Cells and Endothelial Progenitors and Capillaroscopic Patterns in Patients with Systemic Sclerosis. J Dermatolog Clin Res 8(1): 1130.
ABBREVIATIONS
SSc: Systemic Sclerosis; CECs: Circulating Endothelial Cells; EPCs: Endothelial Progenitor Cells; NVC: Naifold Videocapillaroscopy
INTRODUCTION
ystemic sclerosis (SSc) is a rare systemic autoimmune disease characterized by endothelial cell dysfunction and fibrosis of skin and internal organs [1]. The pathogenetic mechanisms involve three components represented by vascular dysfunction and injury, immune disregulation and increased secretion of collagen by fibroblasts.Recently the number of both circulating endothelial cells (CECs) and endothelial progenitor cells (EPCs) has been identified as a marker of vascular damage in a variety of different disorders, including malignancy, cardiovascular diseases and autoimmune disorders such as SSc [2-4]. CECs are terminally differentiated cells with a low proliferative potential, rarerly detectable in healthy subjects. EPCs are bone marrow-derived cells; they may differentiate into mature CECs contributing to re-endothelization, neovascularization and normalization of endothelial function at the side of any vascular injury. As a consequence a reduced number of EPCs has been linked to endothelial dysfunction as well as increased risk foratherosclerosis and cardiovascular morbidity and mortality, while an increased in their number and function have been related to vascular repair.
Endothelin-1 (ET-1), a potent vasocostrictor and profibrogenic factor mainly produced by endothelial cells and fibroblasts, has been found to be elevated in the serum of SSc patients. Preliminary data indicate that ET-1 may be involved in the regulation of EPCs recruitment and that high ET-1 levels are associated with higher number of circulating EPC [5,6].
Bosentan, a non selective ET-1 receptor antagonist, has been shown to induce long-term clinical improvement in various vascular conditions, including ischemic ulcers and pulmonary artery hypertension secondary to SSc. Since plasma levels of ET1has been recently associated to high number of EPC in patients affected by diabetes [6], and since Bosentan mayinterfere with vascular damage in SSc, we aimed at studying the effects of dual ET-1 receptor blockade on CECs and EPCs in patients with SSc.
METHODS
Patients: clinical assessment
We enrolled 20 patients affected by SSc: 7 patients without and 13 patients with active digital ulcers; 11 patients were affected by the diffuse cutaneous form and 9 by the limited cutaneous form of the disease. Ten age- and sex- matched healthy donors were enrolled as controls.The study has been structured as an observational longitudinal trial and was approved by the local ethical committee;written informed consent was obtained from all the partecipants to the study.
All the patients were treated with platelet inhibitors, calcium channel blockers, statins and monthly infusion of iloprost. Moreover all patients either affected by limited or diffuse form of the disease, experienced skin ulcers at least in the previous 3 months and a subgroup of all subjects displayed active digital ulcers. Treatment with Bosentan was started either according to the recent hystory of digital ulcers or to the presence of active skin ulcers.None of the included subjects was treated with immunosuppressive drugs. We considered the presence of PAH as an exclusion criteria, since of the possible interference of this organ involvement both on CECs and EPCs numbers and NVC patterns or capillaries destructuration. Data regarding all clinical features and organ involvement as well as current therapy are summarized in Table 1.
Table 1: Data regarding clinical features and therapy of all enrolled patients
| Characteristics | |
| Age | 48,3 ys (28-76 years)* |
| Sex (male/female) | 1/19 |
| Disease’sduration | 6,7 ys (1,5-23 years)* |
| Disease’sform (diffuse/limited) | 11/9 |
|
Organinvolvement -Active skinulcers -Interstitiallungdisease -Pulmonaryarterialhypertension -Gastro-intestinalinvolvement |
13/20 7/20 0/20 19/20 |
| mRSS | 28,6 ± 7,6 § |
|
Therapy -platelet inhibitors (acetylsalicylic acidclopidogrel) -calciumchannelblockers (nifedipineamlodipine) -statins -iloprost infusion (once upon month) -immunosuppressivedrugs |
20/20 (17/20- 3/20) 20/20 (15/20- 5/20) 20/20 20/20 0/20 |
|
Drop out - discontinuation due to hepatotoxic effect of bosentan - interruption for columnar oedemas of the lower limbs with weight gain |
6/20 4/6 2/6 |
|
*data is expressed as mean value while the range is included in brackets § data is referred to the parameter at the enrollment visit |
|
Fourtheen patients were followed up for 12 months after the beginning ofbosentan. Clinical evaluations before therapy and after 1, 3, 6, 9 and 12 months from the beginning of treatment were performed. Six patients dropped-out because of adverse events related to Bosentan therapy, such as hepatotoxic effect or columnar oedemas of the lower limbs with weight gain exceeding 5 kg, experienced respectively in the first 3 or 6 months. These patients were followed for another month after treatment’s discontinuationwith the same clinical, laboratoristic and instrumental schedule applied for the study.
Nailfold videocapillaroscopy
All patients and healthy controls were evaluated by nailfold videocapillaroscopy (NVC) at baseline and in the subgroup of patients NVC was repeated at each clinical evaluation after 1, 3, 6, 9 and 12 months from the beginning of Bosentan and after one months from Bosentan discontinuation in dropped-out subgroup patients.
Images from nailfold of each finger included from second to fourth of each hand were obtained and analyzed from a single investigator by using an optical probe videocapillaroscope (Videocaps, DS MediGroup, Milan-Italy). Four consecutive images for each fingers were analyzed. Capillaroscopy pattern was defined as normal, early, active or late for each subject; we decided to semi-quantitatively scored capillary loss, dilated, giant or ramified capillaries [7,8].
Detection of CECs and EPCs by flow cytometry
Blood samples collected in EDTA using a Vacutainer system (Becton Dickinson, NJ, USA) were drawn from patients at each clinical control and from healthy donors at the enrollment. CECs and EPCs were directly detected in whole peripheral blood in EDTA by “lyse-no-wash” method by FACS analysis in double platform, combining the flow-cytometrically assessed percentage of cells and the white blood cells (WBC) count assessed using a haematology cell analyser as previously described [4,9,10].
Statistical analysis
Distribution of continuous variables was expressed as means ± standard deviation, while non parametric test, such as KruskalWallis test, and linear regression analysis was performed for all the other variables. In particular comparison of CECs and EPCs levels between healthy controls and patients affected by SSc were performed by T-test and Pearson test. Correlations between CECs and EPCs number before and after bosentan therapy were assessed with a non parametric test (Wilcoxon test). P-value<0,05 was considered statistically significant. All the calculations were performed with SPSS 21.0 statistical package (SPSS Inc., Chicago, IL, USA).
RESULTS
Patients presented different organ involvement, particularly all presented hystory of ulcers or active digital ulcers and skin fibrosis, as mRSS demonstrated. Gastrointestinal involvement was the most frequent complication andinterstitial lung disease was present only in a small percentage of patients and with low severity, according to the information and radiological score obtained with chest high-risolution computed tomography (HRCT). During all the observation period no changes in both internal organ involvement or skin fibrosis were observed as demonstrated by the clinical and instrumental follow-up and the stability of mRSS over the time.
Accordind to the NVC pattern, we observed a normal pattern in all healthy controls while patients were divided in 3 different subgroups, since 5 (25%) of them displayed early pattern, 12 (60%) active and 3 (15%) of them a late pattern. Skin fibrosis showed a strong relation to NVC pattern, since mRSS with value included from 6 to 14 is related to active pattern and value over 14 is strongly associated to late pattern detection. The presence of active DUs is strongly associated to an active-NVC pattern, while a positive hystory of DUs is better associated to an early pattern. Otherwise patients with a late-NVC pattern were more likely to have both active DUs and mRSS > 14.
Independently to the NVC pattern, we analized the number of capillary loss such as dilated, giant or ramified capillaries with the aim to evaluate any possible relation between NVC appearance and clinical features according to the recent literature [11-13]. In particular the decrease in capillary density as well as the presence of capillary loss is associated to the presence of active DUs while the number of ramified capillaries and avascular area are higher in case of pulmonary involvement. During the observation time and the follow-up after drop-out, we didn’t observed any significant variation of NVC-pattern. In the 8 subjects that experienced the complete resolution of digital ulcers we observed a slowly increase in capillary numbers at the 9th month ant 12th month control respectively in 3 and 8 patients. We also observed changes in ramified capillaries’numbers in a subgroup of patients, since 3 subjects experienced both improvement of DUs and increase in remified capillaries since the 6th month of treatment without any statistically relevant significance.
CECs were defined as CD45 negative, CD146/CD31/CD34 positive and CD133 negative. EPCs are greater than CECs and are CD146/CD31 negative, CD34/CD133 positive, CD45 low positive and VEGFR2 positive [9]. Evaluation of CECs and EPCs by flow-cytometry showed that the number of CECs and EPCs were significantly higher in SSc patients than in controls (680,5 ± 162,4 vs 22 ± 17,2 -p<0,001- and 38,6 ± 29,2 vs 2,1 ± 1,3 -p<0,001- respectively) and that among patients, both CECs and pEPCs numbers were higher in patients with skin ulcers than in those without ulcers (959,2 ± 202,7 vs 642 ± 104,9 -p=0,05- and 79,2 ± 11,9 vs 58,3 ± 14,6 -p=0,04), as already reported [4]. We did not found significant correlation between cell count and other clinical parameters such as cutaneous form, modified Rodnan Skin score (mRSS) or internal organ involvement.
According to NVC-pattern, the presence of active pattern is associated to both CECs and EPCs higher levels whereas EPCs significantly decreased according to a late-NVC pattern detection. Capillary loss, expressed as percentage included from 20 to 50%, is associated to high levels of CECs compared to percentage respectively < 20% or > 50%, whereas the lower level in EPCs is detectable in subjects with capillary loss > 50% and with avascular areas. In a multivariable analysisperformed with multiple linear regression including CECs and EPCs, mRSS, capillary loss, NVC-pattern and presence of DUs, lower EPCs counts and capillary loss >50% are independently associated to late-NVC pattern as well as higher CECs number and active DUs with active NVC-pattern.
CECs and EPCs significally increased after 3, 6, 9 and 12 months of therapy (Figure 1),
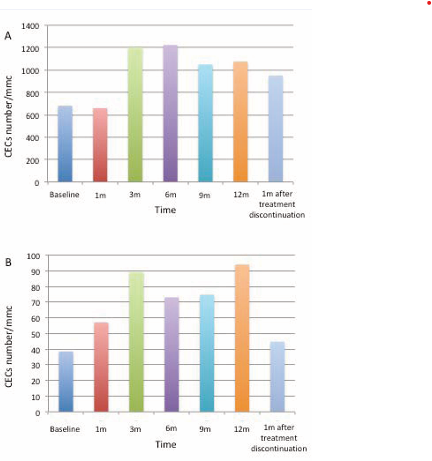
Figure 1 Data presented in Table 2 regarding CECs (panel A) and EPCs (panel B) numbers are represented as histograms. Data are expressed as mean value/mmc.
while there was no difference in cells counts from baseline and the first months of therapy or after 1 month from treatment suspension (Table 2). Moreover FACS analysis showed an increased EPCs positivity for VEGFR2 during bosentan treatment (58% vs 33%).
Table 2: Statistical analysis of CECs and EPCs number before and after the beginning of bosentan treatment.
| Time | n. patients | CECs/mmc | EPCs/mmc | p value | |
| Baseline | 20/20 | 680,5 ± 132,4 | 38,6 ± 29,2 | CECs | EPCs |
| 1 months | 18/20 | 659,9 ± 381,8 | 57,1 ± 56,9 | 0,8 | 0,3 |
| 3 months | 15/20 | 1201 ± 516,9 | 89,1 ± 69,6 | 0,003* | 0,03* |
| 6 months | 14/20 | 1224,1 ± 691 | 73,1 ± 46,5 | 0,01* | 0,02* |
| 9 months | 14/20 | 1050,5 ± 545,5 | 74,8 ± 30,0 | 0,04* | 0,05* |
| 12 months | 14/20 | 1075,5 ± 615,9 | 94,0 ± 32,5 | 0,04* | 0,03* |
| 1 month after stop | 6/20 | 951,3 ± 534,7 | 44,8 ± 28,2 | 0,1 | 0,6 |
| p value has been calculated comparing each time point with the baseline cell count. | |||||
DISCUSSION AND CONCLUSIONS
We have quantified CECs and EPCs in SSc patients and healthy controls by FACS analysis demonstrating a significant difference in the two groups since cellcount is significantly higher in patients.Moreover within patients higher numbers of CECs and EPCsare detectable in patients with a more aggressive form of SSc such as subjects displaying digital ulcers and active NVC patternaccording to the potential role suggested for EPCs as an endogenous repair mechanism of vascular damage [14]. sOn the contrary low levels of EPCs are associated to capillary loss superior than 50% and late NVC pattern, suggesting losting in efficacy of repair machinery. In this contest low EPCs levels in subjects with DUsmay be explained if we considered that skin ulcers are both associated to vascular damage and fibrosis and particularly in our population we observed association of DUs, high mRSS and late NVC pattern, suggesting a prevalence of fibrosis with low vascular repair potential. In this contest we can hypothize that NVC is an useful instrument to monitorize disease activity, since it’s related not only to the presence of DUs and skin fibrosis but also to CECs and EPCs mobilization.
Finally we found that treatment with bosentan leads to a significant enrichment in both CECs and EPCs and that the enrichment is stable after the first month and than during all the study duration.These findingsare in contrast with those reported by Jung et al. [6], who describes an increased levels of EPCs related to high ET-1 plasma levels in patients affected by diabetes without any number modification after a 4 week treatment with bosentan. In SScET-1 levelsare persistently high and do not show consistent modification during treatment with Bosentan in respect to baseline [15]. Moreover ET-1 levels seem to be strongly associated with the number of EPCs and as a consequence to a positive effects of Bosentan treatment.
Again we observed that Bosentan therapy increased both CECs and EPCs and that these cells display higher level of VEGFR2. We can therefore hypothesize that treatment with Bosentancan act by inducing higher expression of VEGFR2 and that long therapy is needed to persistently observe an increase of EPCs leading to vascular repair. VEGF is a potent angiogenic mitogen playing a crucial role in angiogenesis under various pathophysiological conditions, included SSc. In SScVEGF serum levels is not only strongly related to the onset of the disease with lower levels in the early stage and increased levels in the end stage, but also to NVC pattern and with decreased capillary density and angiogenesis [12,16,17].
We have recently demonstrated by gene-array approach that in SSc VEGF is strongly down-regulated and that Iloprost infusion lead to an increase in VEGF gene expression levels possibly explaining the EPCs mobilization in an attempt to repair the vascular damage [4]. Is it possible to hypothize that long-term beneficial effects of bosentan therapy in SSc may be correlated also to the mobilization of EPCs expressing higher VEGFR2 in a possible attempt to favour the repair of endothelial cell damage and the vasculogenesis.
In conclusion our data point out a role of both CECs and EPCs a markers of the disease and evidence a correlation between these markers and clinical features. Moreover the presence of correlation between CECs and EPCs and not only NVC pattern but also NVC appearance, such as capillary density and change in dilated, giant or ramified capillaries, highlight the role of videocapillaroscopy in the monitoring of the disease and as a predictor of its evolution or course.
Again detection of increased levels of CECs and EPCs as an effect of Bosentan treatment may support the hypothesis of a possible role of ET-1 receptor antagonists in the modulation of vascular repair in SSc.









































































































































