Evaluation of a Combination of Retinol, Hydroxyl-Pinacolone Retinoate (Retinsphere), An Antibacterial Agent (BIOPEP.15), Salicylic Acid and Vitamin E in Subjects Suffering from Predominant Comedonal Acne-An Italian multicenter Prospective Open study
- 1. Department of Dermatological Sciences, University of Milan, Fondazione I.R.C.C.S, Italy
- 2. Service of Dermatology Ugento, Lecce, Italy
- 3. Service of Dermatology Hospital of Prato, Italy
- 4. Clinica Dermatological Hospital, San Martino University of Genova, Italy
- 5. Department Sciences and Medical and Surgical Biotechnology, Sapienza University of Rome, Italy
- 6. Medical Direction Difa Cooper, IFC Group, Caronno Pertusella, Italy
Abstract
Background: Topical retinoids are considered the first-line approach in the treatment of comedonal or mild inflammatory acne. However local tolerability of topical retinoids could be a limiting factor regarding their widespread clinical use in this setting. A new topical formulation containing two vitamin A derivatives (retinol and hydroxypinacolone retinoate, carried in a patented glycospheres system RetinSphere®), an antimicrobial peptide (Biopep.15), salicylic acid and vitamin E has been recently developed (Biretix gel ULTRA) (BR).
Study aim: We evaluated in a prospective multicenter open study the efficacy and tolerability of BR in a real-life clinical condition. BR was applied one/twice daily for a total of 90 consecutive days. Subjects and methods: In a prospective 12-week trial we enrolled a total of 100 subjects (32 men and 68 women, mean age 23 years) with predominant comedonal facial acne. The primary outcome of the study was the evolution of non-inflammatory (NI-L) (comedons) and inflammatory lesions (IL) in comparison with baseline and after 30, 60 and 90 days. Secondary outcome was the evaluation of skin irritation (erythema E, desquamation D, burning sensation B, and skin xerosis X) using a semi-quantitative score from 0(no symptom) to 3(relevant symptom). In addition a global skin tolerability score (E+D+B+X: maximum score 12) was also calculated.
Results: All subjects concluded the study. At baseline mean ± SD of NI-L were 30 ± 14. In comparison with baseline, NI-L number was reduced significantly to 22 ± 11 at day 30, to 16 ± 8 at day 60 and to 12 ± 8 at the end of the study period (a -60% vs. baseline) (p=0.0001; Wilcoxon Test). At baseline, the mean number of IL was 10 ± 4. Active treatment significantly reduced IL to 7± 4 at day 30, to 5 ± 4 at day 60 and to 3 ± 3 at day 90 (a -70% vs. baseline) (p=0.0001; Wilcoxon Test).The global skin tolerability score was 1.14 at day 30 and 0.42 at day 60. At the end of the study the mean global skin tolerability score was 0.26. Xerosis (very mild/mild) was reported by 24 (24%) of subjects after 30 day and by only 7 (very mild) (7%) at the end of treatment period.
Conclusion: BR gel has shown to be a very effective and well tolerated treatment of mild to moderate acne reducing both non inflammatory and inflammatory acne lesions. This formulation has also good skin tolerability. BR could be considered a suitable first-line treatment for predominant Comedonal acne.
Keywords
Comedonal acne, Antibacterial agent, Retinol, Hydroxyl- Pinacolone retinoate
CItation
Veraldi S, Barbareschi M, Alessandrini G, Cardinali C, Gimma A, et al. (2016) Evaluation of a Combination of Retinol, Hydroxyl-Pinacolone Retinoate (Retins phere), An Antibacterial Agent (BIOPEP.15), Salicylic Acid and Vitamin E in Subjects Suffering from Predominant Comedonal Acne-An Italian multicenter Prospec tive Open study. J Dermatolog Clin Res 4(3): 1072.
INTRODUCTION
Acne is a chronic inflammatory disease of the pilosebaceous units of the face, neck, chest, and back [1]. It is the most common skin disorder occurring worldwide, with an estimated prevalence of 70-87% [2]. Oral treatments (antibiotics or systemic retinoids) are commonly used in subjects with acne. Recently a consensus work [3] indicates that subjects with predominant comedonal acne should initially be treated with topical retinoids. However this strategy could have some limitations [4]. Subjects treated with topical retinoids could complain of adverse events such as skin irritation and xerosis [5]. A new topical formulation containing 0.15% of two vitamin A derivatives (retinol and hydroxypinacolone retinoate, carried in a patented glycospheres system: RetinSphere®), an antimicrobial peptide (BIOPEP-15), salicylic acid 0.5% and vitamin E (BR) has been recently developed (Bioretix gel ultra, Difa Cooper, IFC Group, Caronno Pertusella, Italy). The RetinSphere® is an innovative technology which can acts as a penetration enhancer allowing a slow release of the active principles, improving the chemical stability and at the same time the bioavailability [6]. This composition, from a theoretical point of view, suggests that this product could be particular effective in the treatment of comedonal and mild inflammatory acne with at the same time good skin tolerability. So far no real-life data on a large sample population are available evaluating the efficacy and tolerability profiles of this product.
STUDY AIM
We evaluated the clinical efficacy of BR in a real life condition carrying out a multicenter 12-week prospective open trial.
SUBJECTS AND METHODS
Study design
The present study was a prospective open trial. The study was carried out in 5 outpatient third level acne Clinics in Italy. Study was performed between January 2015 and April 2016.
Subjects
We enrolled a total of 100 subjects (men and women, mean age 21 years) after their written informed consent. Main entry criteria were the presence of mild to moderate facial acne (predominant comedonal acne as defined by Gollnick et al.). Main Exclusion criteria were: severe forms of acne requiring systemic treatments; other severe skin conditions; use of topical acne medications such as tretinoin, benzoyl peroxide or topical antibiotics within 2 weeks prior the enrollment in the trial; use of oral antibiotics within 30 days; use of systemic corticosteroids within 4 weeks and a Body Mass Index >30. The product was applied once or twice daily (in the morning and in the evening) in consideration of the severity of acne at baseline (65 subjects were treated with once daily schedule and 35 with a twice daily application). The total amount of product applied was in average 2 Finger Tip Units per application in order to cover the affected areas. At baseline visit, acne was graded using the Global Acne Grading System (GAGS) [7].
Study outcomes
The primary outcome of the study was the evolution of noninflammatory lesions (comedons) and inflammatory lesions (papules and pustules) in comparison with baseline. Secondary outcome was the evaluation of skin tolerability. In particular safety and tolerability were assessed through evaluations of treated areas tolerability and adverse events. At each visit (after 30, 60 and 90 days), the investigator rated erythema (E), desquamation (D) burning (b) and xerosis (X), on the basis of a semi-quantitative scale ranging from 0 (none) to 3 (severe). In addition global skin tolerability score (E+D+B+X) was also calculated (maximum score value: 12). Study protocol and patient information sheet were approved by each local Investigational Review Boards.
Statistical analysis
All statistical analyses were performed using SPSS statistical package Version 13. The Shapiro–Wilk test was used to evaluate the normal distribution of continuous variables. Two-tailed Wilcoxon paired tests were applied to compare baseline levels with values at each time point evaluation at the end of study period. The analysis was based on the intention-to-treat principle and involved all patients who were enrolled. A p value <0.05 was considered statistically significant.
RESULTS
Table shows the subjects’ baseline characteristics. All the enrolled subjects concluded the study. At baseline, mean ± SD of NI-L were 30 ± 14. In comparison with baseline, NI-L number was reduced significantly to 22 ± 11 at day 30, to 16 ± 8 at day 60 and to 12 ± 8 at the end of the study period (day 90) (a -60% vs. baseline) (p=0.0001; Wilcoxon Test). At baseline the mean number of IL was 10 ± 4. Active treatment significantly reduced IL to 7 ± 4 at day 30, to 5 ± 4 at day 60 and to 3 ± 3 at day 90 (a -70% vs. baseline) (p=0.0001; Wilcoxon Test). [Figures 1,2] show the evolution of NI-L and IL during the study period.
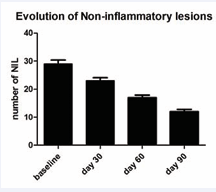
Figure 1 Evolution of Non-Inflammatory lesions and use of BR. A statistical significant reduction of lesions numbers was observed starting from day 30 in comparison with baseline value. At day 90 mean lesion number was 60% less than baseline value.
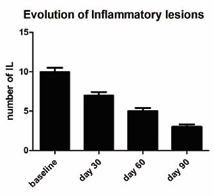
Figure 2 Evolution of Inflammatory lesions and use of BR. A statistical significant reduction of lesions numbers was observed starting from day 30 in comparison with baseline value. At day 90 mean lesion number was 70% less than baseline value.
The global skin tolerability score was 1.14 at day 30 and 0.42 at day 60. At the end of the study the mean global skin tolerability score was 0.26. Xerosis (very mild/mild) was reported by 24 (24%) of subjects after 30 day and by only 7 (very mild) (7%) at the end of treatment period. Peeling (very mild/mild) was reported by 20 (20%) subjects at day 30 and by 7 (7%) at day 90. No differences regarding efficacy or skin tolerability were observed in relation of number of daily applications (once vs. twice).
Table 1: Subjects Characteristics at baseline.
| BR group | |
| Number | 100 |
| Men/Women | 32/68 |
| Age,years, mean (SD) | 22(6) |
|
Phototype (Fitzpatrick) I II |
29 71 |
|
Acne Severity Mild, n (%) Moderate n (%) |
66 (66%) |
| 34 (34%) | |
| GAGS score, mean(SD) | 9.8 (6.9) |
| NB: Mild Acne: GAGS score: from 1 to 18; Moderate Acne: GAGS Score: from 19 to 30. | |
DISCUSSION
Acne is a chronic inflammatory disease of the pilosebaceous unit resulting from hormone-induced increased sebum production associated with altered keratinization, inflammation, and bacterial colonization of hair follicles on the face, neck, chest, and back by P. acnes [8]. Acne is a common skin condition [9]. In the present study we evaluated in a real-life condition the clinical efficacy of a gel formulation containing two vitamin A derivatives (hydroxypinacolone retinoate and retinol) carried in glycospheres (RetinShpere), an antimicrobial compound (Biopep-15) and a keratolytic agent in mild to moderate acne. Hydroxypinacolone retinoate is a retinoic acid ester and thus carries out retinoid activity while at the same time ensuring greater skin tolerability. Glycospheres help enhance delivery of the retinol. Vitamin A derivatives are commonly used in the treatment of acne, both topically and orally [10]. Oral treatment with retinoid is in general limited for severe forms of acne [11]. Topical retinoids are commonly used in mild and moderate acne [12]. Retinoids are the core of topical therapy for acne in view of their potent comedolytic and anti-inflammatory actions [13]. However their use in acne could be limited by side effects [14]. The use of topical retinoids is commonly associated with skin dryness peeling, erythema and irritation [15]. In addition some formulations of retinoids are not photo stable and should be applied in the evening, avoiding sun exposure. Hydroxypinacolone retinoate is a new synthetic ester of 9-cisretinoic acid. Retinol is one of the best known cosmeceutical forms of vitamin A [16]. The Retinsphere technology is capable to act as a penetration enhancer allowing a slow release of the active principles, improving the chemical stability and at the same time the bioavailability. BIOPEP-15 is a polypeptide with an anti P. acnes activity [17]. Salicylic acid is a comedolytic agent that could be used in 0.5% to 2% strengths as an over the counter product. This product is commonly used in the therapeutic strategies of mild to moderate acne in general combined with other products [18]. Vitamin E is the major naturally occurring lipid-soluble nonenzymatic antioxidant protecting skin from the adverse effects of oxidative stress [19]. The topical product we have evaluated in this trial is therefore formulated with 4 different active principles which could act at different levels of the pathogenesis of acne. In our trial we have also shown that this formulation has a good local tolerability. This could be due to characteristics of the specific composition of the gel (presence of vitamin E, the combination of retinoids, and the delivery system). Some limitations should be considered in evaluating our results. First, this study was an open uncontrolled trial. However we decided to perform a reallife experiences in a relative high number of subjects and in addition we believe that the external validity of the present study could be considered good in consideration that inclusion and exclusion criteria were not particularly strict, offering therefore an effective generalization of the results obtained.
CONCLUSION
BR gel, containing two vitamin A derivatives, an antimicrobial peptide, salicylic acid and vitamin E has shown to be a very effective treatment of mild to moderate acne reducing both noninflammatory and inflammatory lesions. This formulation has also very good skin tolerability. Therefore BR could be considered a suitable first-line treatment for predominant comedonal acne.
COMPETING INTERESTS
MM is an employee of Difa Cooper. All others authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
MB, AP and SV had the original study idea and participated in its design and coordination. MM helped regarding study design, protocol definition, data collection and analysis and manuscript preparation. GA, NS, CC, AP, AG and MB carried out the patients’ selection and follow up visits. All authors read and approved the final manuscript.
ACKNOWLEDGEMENT
The study was funded by independent non-profit source. Study drugs were supplied by Difa Cooper, Italy. No potential conflicts of interest relevant to this paper were reported.
REFERENCES
1. Thiboutot DM. Overview of acne and its treatment. Cutis. 2008; 81:3-7.
7. Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997; 38: 416- 418.
8. Williams HC, Dellavalle RP, Garner S. Acne vulgaris. The Lancet. 2012; 379: 361-372.
13.Leyden JJ. Retinoids and acne. J Am Acad Dermatol. 1988; 19: 164-168.
15.Healy E, Simpson N. Acne vulgaris. BMJ: British Medical Journal. 1994; 308: 831.
17.Bojar DJR. Fitzgerald, Efficacia anti-microbica di BIOPEP·15 valutata su 24 ceppi di Propionibacteriumacnes. Evocutisplc. EVO-0406 Oct 2012
19.Nachbar F, Korting HC. The role of vitamin E in normal and damaged skin. J Mol Med. 1995; 73: 7-17.









































































































































