IgG or IgA Pemphigus: Report of a Rare Variant of Atypical Pemphigus and a Review of the Literature
- 1. Departments of Dermatology, Wake Forest University School of Medicine, USA
- 2. Departments of Pathology, Wake Forest University School of Medicine, USA
Abstract
First described as a distint clinical entity in 1994, IgG/IgA pemphigus is a rare type of atypical pemphigus with a variety of clinical, histological, and immunological manifestations. Only 21 cases of this disease have been reported in the literature to date. We report a case of IgG/IgA pemphigus in a 54-year old female in which the diagnosis was delayed due to negative direct immunofluorescence results following two separate biopsies. The clinical and histological findings associated with this rare form of pemphigus will be discussed along with a review of the literature.
Keywords
Pemphigus , Atypical pemphigus , Direct immunofluorescence , Desmoglein , Desmocollin , Intraepidermal neutrophils
Citation
Watkins C, West C, Kosari P, Ali S, Sangüeza OP, et al. (2014) IgG/IgA Pemphigus: Report of a Rare Variant of Atypical Pemphigus and a Review of the Literature. J Dermatolog Clin Res 2(1): 1011.
INTRODUCTION
Pemphigus is an autoimmune blistering disease characterized by antibodies that target intercellular components of the epidermis. Several variants exist and can be broadly classified into typical and atypical pemphigus disorders. IgG/ IgA pemphigus is a relatively new form of atypical pemphigus characterized by deposits of both IgA and IgG antibodies in the epidermis. Although a role for IgA in pemphigus was identified in 1987, the distinct disease entity was first described by Chorzelski et al. in 1994 [1]. The true incidence is unknown and to our knowledge, only 21 cases have been reported in the literature to date [2]. The immunopathologic and clinical features may vary, however, vesiculopustular eruptions demonstrating neutrophilic acantholysis upon biopsy is commonly observed [3]. Diagnosis must be confirmed with direct immunofluorescence identification of both IgG and IgA antibodies within the epidermis.
We present a case of IgG/IgA pemphigus and discuss the associated clinical, histological and immunological findings described in the literature. The clinical presentation of IgG/ IgA pemphigus may vary significantly, therefore, proper biopsy technique and immunofluorescence testing is necessary for diagnosis and will also be discussed.
METHODS
Review of the medical records provided information about patient history, biopsies and histological examination, including direct and indirect immunofluorescence. Direct immunofluorescence (DIF) was performed using polyclonal rabbit anti-human IgG, IgA, IgM and C3 Complement. Indirect immunofluorescence (IIF) was performed at dilutions of 1:10, 1:20, 1:40 with the patient’s serum and monkey esophagus as the substrate using polyclonal rabbit anti-human IgG. A review of the literature was performed using PubMed/MeSH Database search. Available case reports and current review articles were investigated to provide up-to-date information about IgG/IgA pemphigus. Patient consent was obtained for photographs and appropriate clinical procedures.
CASE REPORT
A 54 year-old Caucasian female was referred to our dermatology clinic for a 3-month history of a blistering disease associated with pruritis. Before her presentation to our clinic, she was seen and treated by two other dermatologists. Diagnoses considered included primary vesiculobullous disorders such as pemphigus vulgaris, pemphigus foliaceous, dermatitis herpetiformis and linear IgA bullous dermatosis. Other clinical concerns included subcorneal pustular dermatosis, acute generalized exanthemous pustulosis and bullous impetigo. Subsequently, she underwent a skin biopsy, by each dermatologist, separated by one month apart. The initial specimen was obtained from the left posterior arm and showed a spongiotic dermatitis with acantholysis and an intraepidermal pustule. The second biopsy was from the abdomen and showed a subcorneal vesicular dermatitis with eosinophilic spongiosis. Direct immunofluorescence (DIF) was also performed with each biopsy and was negative in both cases.
On presentation to our clinic, she described blisters that began on the upper back and gradually progressed to the trunk and extremities. The lesions were pruritic and sore. She denied mucosal involvement. She was treated with multiple courses of high dose oral prednisone which controlled the disease, although blisters rapidly reappeared with any attempt to taper her off corticosteroids. No new medications, travel, or recent exposures were elicited on history. Family history was negative for blistering or auto-immune disorders. She was otherwise healthy and review of systems was otherwise negative.
While on a daily dose of prednisone 60 mg, physical examination revealed numerous erythematous papules and plaques with overlying erosions and small vesicles located on the trunk and proximal extremities (Figure 1).
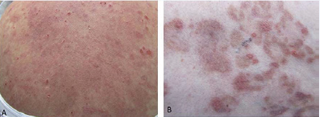
Figure 1 IgG/ IgA Pemphigus. (A) Numerous erythematous papules and plaques with overlying erosions and small vesicles involving the trunk and proximal extremities. Serum crust is present. (B) Magnified view of papules and plaques.
Some lesions were arciform and annular in arrangement with overlying serum crusting. Her conjunctiva and oral mucosa were without lesions on initial presentation. A complete blood count, comprehensive metabolic panel, and urinalysis were within normal limits except for mild leukocytosis (13.0 K/uL) with an elevated neutrophil count (11.3, N 1.6-7.3) and mildly elevated liver enzymes (AST 110 U/L, ALT 178 U/L). Anti-nuclear antibody titer was positive at 1:160.
Though there were two previous pathological reports of negative DIF testing, clinical suspicion for an autoimmune blistering disorder was high. Sneddon-Wilkinson disease was also considered. Two perilesional 4-mm punch biopsies were performed on the patient’s back. Routine histopathological examination was significant for an acantholytic dermatitis with neutrophilic aggregates in the superficial epidermis (Figure 2).
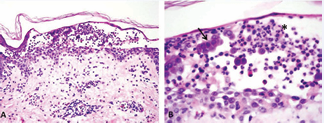
Figure 2 IgG/IgA Pemphigus. (A) Intraepidermal vesicle with acantholysis in the superficial epidermis and neutrophilic aggregates. H&E stain, 10x magnification. (B) Magnified view demonstrating intact acantholytic keratinocytes (black arrow) and neutrophilic aggregates (asterisk). H&E stain, 40x magnification.
Direct immunofluorescence was positive for intercellular deposits of IgG and IgA in the epidermis (Figure 3).
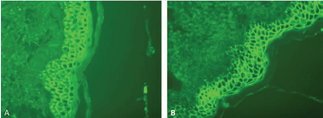
Figure 3 IgG/IgA Pemphigus. Direct immunofluorescence testing for IgA and IgG performed following a perilesional biopsy. (A) Intercellular deposits of IgA. (B) Intercellular deposits of IgG showing greater staining intenstity with IgG compared to IgA. Intercellular C3 deposition is seen in the lower portion of the epidermis. Direct Immunofluoresence stain, 40x magnification.
C3 was also positive in the lower portion of the epidermis. Subsequent serum indirect immunofluorescence (IIF) on monkey esophagus did not demonstrate intercellular fluorescence with IgG or IgA.
The patient was continued on 60 mg of prednisone daily while starting systemic dapsone and colchicine. Her systemic corticosteroids have been slowly tapered and she has achieved complete control of her symptoms with dapsone 100 mg daily and colchicine 0.6 mg twice daily. Of note, during her treatment course, she developed oral erosions involving the posterior pharynx.
The patient has had all age appropriate cancer screenings. Upon her diagnosis, a chest X-ray and serum protein electrophoeresis were obtained and found to be within normal limits. She continues to be followed closely in our clinic.
DISCUSSION
IgG/IgA pemphigus is considered a rare atypical variant of pemphigus vulgaris. It was first described in 1994 and is a relatively new clinical-pathological disease entity [1]. Only 21 cases have been reported to date. Five cases are incompletely described and not included in the table. Table 1 summarizes data available for the remaining 16 cases.
Table 1: Reported Cases of IgG/IgA Pemphigus.
| Patient | Clinical | DIF |
Antibody targets |
Histology | Treatment | Malignancy | References |
| 81 yo emale | Vesiculobullous erythema, trunk and extremities | IgG IgA C3 | Desmoglein1 | Acantholytic cells, neutrophil infiltration | Dapsone | Gallbladder carcinoma | [7] |
| 33 yo female | Vesiculobullous erythema, pustules | IgG IgA | Desmoglein1 | Superficial bullae with neutrophil infiltration | Dapsone, Prednisone | Ovarian carcinoma | [6] |
| 25 yo female | Vesicles, annular erythematous plaques with erosions | IgG IgA | Desmoglein1 | Acantholytic cells, neutrophilic intraepidermal pustules | Topical clobetasol dipropionate | -- | [2] |
| 70 yo male | Erythematous annular plaques, vesicles and pustules | IgG IgA | Desmoglein1 | Subcorneal pustules and vesicles, acantholytic cells | Acitretin, methylprednisolone | Pancreatic carcinoma | [2] |
| 43 yo female | Flaccid bullae, crusted erosions, oral mucosa involvement | IgG IgA C3 | Desmoglein1 Desmoglein3 | Acantholytic bullae with lymphocytic and neutrophilic infiltrate | Oral prednisone, topical betamethasone valerate | -- | [4] |
| 48 yo male | Vesicles, erythematous macules with eroisions | IgG C3 | Desmoglein1 Desmoglein3 (circulating IgA antibodies identified on ELISA) | Dyskeratosis, acantholysis, neutrophilic infiltration, microabscesses | Dapsone | -- | [3] |
| 42 yo female | Annular erythema with peripheral vesicles, erosions with crusting | IgG IgA C3 | Desmoglein1 | Acantholytic bullae, neutrophilic infiltrate | Prednisone, minocycline, topical betamethasone valerate | -- | [5] |
| 39 yo female | Vesicles, pustules, central erosions and crusts, flaccid bullae | IgG IgA | Desmocollin1 | Neutrophilic pustules, acantholysis | Dapsone | -- | [10] |
| 11 yo male | Flaccid pustules, bullae, vesicles and erosions of mucous membranes, conjunctivitis | IgG IgA | Desmoglein3 | Acantholysis, neutrophilic infiltrate | Dapsone, prednisone | -- | [8] |
| 48 yo female | Erosive stomatitis, conjunctivitis, exudative erythema multiforme-like lesions | -- | Desmocollin3 Desmocollin2 BP180 Envoplakin Periplakin | Suprabasilar acantholysis, dense inflammatory infiltrate | High-dose prednisolone. | B-Cell nonHodgkin’s Lymphoma | [13] |
| 5 patients* | Atypical pemphigus features in 3 cases, PV-like appearance in one case and PFlike appearance in one case. | -- | Desmoglein1 Desmoglein3 ** | -- | -- | -- | [18] |
| 78 yo female | Annular erythema, vesicles, pustules, peripheral erosions; esophageal erosions | -- | Desmoglein 1 Desmoglein 3 | Neutrophilic spongiosis with eosinophilic infiltrate, intraepidermal pustules | Prednisolone | -- | [12] |
| 42 yo male | Erythematous plaques with vesicles, pustules and crust; PH-like appearance | -- | Desmoglein 1 Desmocollin 3 | Vesicles with neutrophilic and eosinophilic infiltrate, acantholysis | Prednisolone, dapsone | -- | [11] |
| 60 yo female | Erythematous vesicles and pustules; PF-like appearance | IgG IgA | -- | Acantholysis, neutrophilic blisters | Dapsone | -- | [14] |
| 60 yo female | Vesicles and erythema followed by development of pustules; PF-like appearance | IgG IgA | 140 kD protein | Acantholysis, neutrophilic blisters | Dapsone, then betamethasone | -- | [17] |
| 63 yo male | Erythematous plaques with erosions and crusts | IgG IgA | Desmocollin 1 Desmocollin 2 | Subcorneal acantholytic bullae with neutrophils | Dapsone | Lung cancer | [1] |
|
DIF = direct immunofluorescence; PV = pemphigus vulgaris; PF = pemphigus foliaceus; PH = pemphigus herpetiformis *patient demographics not reported **no specific antigens identified in 3 of the reported cases |
|||||||
As a result, little is known regarding the true incidence and demographics of IgG/ IgA pemphigus. Much variation exists in the clinical, histological, and immunological features adding to the difficulty in diagnosis and evidence based management [3].
The pemphigus spectrum disorders are autoimmune blistering diseases that have antibodies directed against the intercellular surface components of keratinocytes [2,4,5]. The traditional classification of pemphigus includes pemphigus foliaceus, pemphigus erythematosus, pemphigus vulgaris, and pemphigus vegetans [6]. The more recently described and atypical pemphigus spectrum disorders include herpetiform pemphigus, paraneoplastic pemphigus, and IgA pemphigus. IgA pemphigus is further subdivided into subcorneal pustular dermatosis and intraepidermal neutrophilic IgA dermatosis [4,6]. IgG/IgA pemphigus appears to be a unique and separate variant of atypical pemphigus with distinct and variable, clinical and histological manifestations.
IgG/IgA pemphigus is a neutrophilic acantholytic skin disorder that reveals intercellular deposits of both IgA and IgG antibodies within the epidermis [7]. Age of onset is highly variable and ranges from 11 years to 81 years [7,8]. Eleven of the 16 cases (69%) of IgG/IgA pemphigus, including our patient, were reported in women. Interestingly, IgA pemphigus has also been reported more commonly in women [9].
The clinical presentation is variable, sometimes making diagnosis difficult. Our patient had erythematous papules and plaques with vesicles, erosions and crusting (Figure 1).
This presentation, though non-specific, is similar to the clinical findings of other known cases [2,3,5,6,10-12]. The annular configuration of some lesions seen in our patient was reported in at least 4 other patients [2,5,12]. Pustules were also commonly identified. Pruritus and tenderness were also common presenting complaints [3,6,13]. The location of the lesions varied, however, the trunk and extremities were most commonly involved [7,12,14]. Mucosal involvement has been reported [4,13] with esophageal involvement was seen in one patient [12]. No other reports of posterior pharyngeal involvement exist to our knowledge.
Abnormalities in lab work are not common in this disorder unless associated with an underlying malignancy. Our patient had a mild leukocytosis likely due to chronic systemic corticosteroid use. The elevation in liver enzymes could be a sign of an underlying malignancy of hepatic origin [15], however, in our patient further work-up revealed non-alcoholic steatohepatitis. The positive anti-nuclear antibody titer is of indeterminate significance.
A paraneoplastic association with IgG/IgA pemphigus has been suggested [1,2]. The mechanism is unknown, however, anti-desmoglein 1 antibodies were identified in several cases and may be associated [2]. Malignant diseases reported with IgG/IgA pemphigus include lung cancer [1], pancreatic adenocarcinoma [2], carcinoma of the gallbladder [7], lymphoma [13], and ovarian carcinoma [6]. The small number of patients reported with IgG/ IgA pemphigus limits our ability to conclude that a relationship between this rare type of pemphigus and malignancy truly exists although age appropriate cancer screening is indicated.
Histological findings in all reported cases demonstrate a neutrophilic infiltrate [3,4]. Acantholysis is commonly described but not seen in all cases [3]. Nakajima et al. suggest that both dyskeratosis of acantholytic cells and neutrophil infiltration into the epidermis could be considered diagnostic indicators of IgG/ IgA pemphigus [3].
A few target antigens strongly associated with pemphigus disorders have been well established, including desmoglein 3 for pemphigus vulgaris and desmoglein 1 for pemphigus foliaceus [6]. IgG/IgA pemphigus is associated with a variety of antigens. IgA and IgG antibodies targeting both desmogleins 1 and 3 as well as desmocollins 1, 2 and 3 have all been identified to date [2-8,10,13]. This variation in target antigens may account for the clinical and histological variety seen with IgG/IgA pemphigus. It has also been suggested that the coexistence of IgA and IgG antibodies seen in this rare form of pemphigus could be transient, representing a transition period within a single disease or between two pemphigus disorders [6].
The above case described presented some difficulty in diagnosis due to a negative DIF on two separate occasions leading the patient to go undiagnosed for several months even after seeing multiple dermatologists in the past. The initial two biopsies were performed at outside facilities. While speculative, poor biopsy technique or technical difficulties encountered in laboratory processing may have caused the negative DIF results. It is also possible that recent treatment could have caused the repeated false negatives. Finally, misinterpretation by the dermatopathologist in cases with substantial background staining may result in a false negative. The reported sensitivity of DIF in pemphigus disorders is 88% [16]. It is a reliable diagnostic test for pemphigus that becomes positive early in the course of disease and remains positive for long periods after clinical remission [17]. In approximately 50% of cases, IIF detects IgA autoantibodies [18]. Immunofluorescence testing is absolutely necessary for increased detection of those variants of pemphigus, including IgG/IgA pemphigus, that are considered rare [16]. This highlights the importance of proper biopsy technique when autoimmune bullous disorders are suspected.
A perilesional punch biopsy should be obtained. Lesions identified as new by the patient and/or those appearing active on clinical examination should be targeted for biopsy. Biopsy of more than one lesion can be considered, particularly if the lesions have various clinical appearances indicating different stages in the autoimmune disease process. Technical faults and/or treatment-induced changes are often to blame for false negative results, therefore, biopsy should be obtained before beginning the patient on therapy [16]. Our patient had been referred to us already on a daily dose of prednisone 60 mg daily. Moreover, at the time the serum IIF was obtained, the patient had been appropriately treated. This may explain the negative result of the IIF. In addition to immunofluorescence studies, ELISA can be performed to identify the specific IgA and IgG target antigens involved.
The treatment for IgG/IgA pemphigus typically includes a course of high-dose systemic corticosteroids. Oral prednisone (orprednisolone) can be used to induce remission, but in some cases was required for maintenance as well [4-6,8,11-13]. Successful treatment and maintenance therapy with sulfonamides, including dapsone have also been reported [1,3,8,10,11,14,19]. Topical steroids were successful in one case [2], but may also be used in combination with oral agents to help control disease.
CONCLUSION
IgG/IgA pemphigus is a rare and relatively newly identified form of atypical pemphigus. The clinical and histological findings are variable. Erythematous papules and plaques with vesicles, pustules and erosions are typical clinical findings. Intraepidermal neutrophils and acantholysis are the hallmark findings on histology. Definitive diagnosis is obtained via DIF testing which shows intercellular IgA and IgG antibodies directed against the cell surface of keratinocytes. Recent recognition of IgG/IgA pemphigus as a separate disease entity in addition to the small number of reported cases available highlight the need for further studies.
REFERENCES
- Chorzelski TP, Hashimoto T, Nishikawa T, Ebihara T, Dmochowski M, Ismail M, et al. Unusual acantholytic bullous dermatosis associated with neoplasia and IgG and IgA antibodies against bovine desmocollins I and II. J Am Acad Dermatol. 1994; 31: 351-355.
- Santiago-et-Sánchez-Mateos D, Juárez Martín A, González De Arriba A, Delgado Jiménez Y, Fraga J, Hashimoto T, et al. IgG/IgA pemphigus with IgA and IgG antidesmoglein 1 antibodies detected by enzyme-linked immunosorbent assay: presentation of two cases. J Eur Acad Dermatol Venereol. 2011; 25: 110-112.
- Nakajima K, Hashimoto T, Nakajima H, Yokogawa M, Ikeda M, Kodama H. IgG/IgA pemphigus with dyskeratotic acantholysis and intraepidermal neutrophilic microabscesses. J Dermatol. 2007; 34: 757-760.
- Zaraa I, Kerkeni N, Sellami M, Chelly I, Zitouna M, Makni S, et al. IgG/IgA pemphigus with IgG and IgA antidesmoglein 3 antibodies and IgA antidesmoglein 1 antibodies detected by enzyme-linked immunosorbent assay: a case report and review of the literature. Int J Dermatol. 2010; 49: 298-302.
- Oiso N, Yamashita C, Yoshioka K, Amagai M, Komai A, Nagata Y, et al. IgG/IgA pemphigus with IgG and IgA antidesmoglein 1 antibodies detected by enzyme-linked immunosorbent assay. Br J Dermatol. 2002; 147: 1012-1017.
- Kowalewski C, Hashimoto T, Amagai M, Jablonska S, Mackiewicz W, Wozniak K. IgA/IgG pemphigus: a new atypical subset of pemphigus? Acta Derm Venereol. 2006; 86: 357-358.
- Maruyama H, Kawachi Y, Fujisawa Y, Itoh S, Furuta J, Ishii Y, et al. IgA/IgG pemphigus positive for anti-desmoglein 1 autoantibody. Eur J Dermatol. 2007; 17: 94-95.
- Bruckner AL, Fitzpatrick JE, Hashimoto T, Weston WL, Morelli JG. Atypical IgA/IgG pemphigus involving the skin, oral mucosa, and colon in a child: a novel variant of IgA pemphigus? Pediatr Dermatol. 2005; 22: 321-327.
- Ongenae KC, Temmerman LJ, Vermander F, Naeyaert JM. Intercellular IgA dermatosis. Eur J Dermatol. 1999; 9: 85-94.
- Heng A, Nwaneshiudu A, Hashimoto T, Amagai M, Stanley JR. Intraepidermal neutrophilic IgA/IgG antidesmocollin 1 pemphigus. Br J Dermatol. 2006; 154: 1018-1020.
- Kozlowska A, Hashimoto T, Jarzabek-Chorzelska M, Amagai A, Nagata Y, Strasz Z, et al. Pemphigus herpetiformis with IgA and IgG antibodies to desmoglein 1 and IgG antibodies to desmocollin 3. J Am Acad Dermatol. 2003; 48: 117-122.
- Inui S, Amagai M, Tsutsui S, Fukuhara-Yoshida S, Itami S, Katayama I. Atypical pemphigus involving the esophagus with IgG antibodies to desmoglein 3 and IgA antibodies to desmoglein 1. J Am Acad Dermatol. 2006; 55: 354-355.
- Preisz K, Horváth A, Sárdy M, Somlai B, Hársing J, Amagai M, et al. Exacerbation of paraneoplastic pemphigus by cyclophosphamide treatment: detection of novel autoantigens and bronchial autoantibodies. Br J Dermatol. 2004; 150: 1018-1024.
- Miyagawa S, Hashimoto T, Ohno H, Nakagawa A, Watanabe K, Nishikawa T, et al. Atypical pemphigus associated with monoclonal IgA gammopathy. J Am Acad Dermatol. 1995; 32: 352-357.
- Brunton L, Chabner B, Knollman B. Goodman and Gilman’s The Pharmacologic Basis of Therapeutics. McGraw-Hill Professional. 12 edn. Dec. 20, 2010.
- Inchara YK, Rajalakshmi T. Direct immunofluorescence in cutaneous vesiculobullous lesions. Indian J Pathol Microbiol. 2007; 50: 730-732.
- Minz RW, Chhabra S, Singh S, Radotra BD, Kumar B. Direct immunofluorescence of skin biopsy: perspective of an immunopathologist. Indian J Dermatol Venereol Leprol. 2010; 76: 150-157.
- Chan LS. IgA Pemphigus Workup. 2012.
- Ohno H, Miyagawa S, Hashimoto T, Nakagawa A, Watanabe K, Nishikawa T, et al. Atypical pemphigus with concomitant IgG and IgA anti-intercellular autoantibodies associated with monoclonal IgA gammopathy. Dermatology. 1994; 189 Suppl 1: 115-116.









































































































































