Long Term Follow up and Sequential Therapies in Pemphigus: Many Paths to Disease Control and Minimizing Corticosteroid Usage
- 1. St. Joseph Mercy Health System Dermatology Residency Program, Fivenson Dermatology, 3200 W. Liberty Rd, C5; Ann Arbor, MI 48103, USA
- 2. St. Joseph Mercy Health System Dermatology Residency Program, Fivenson Dermatology, 3200 W. Liberty Rd, C5; Ann Arbor, MI 48103, USA
Abstract
Background: Pemphigus management with steroid sparing agents can be prolonged, with cycles of remission and flares common.
Objective: To analyze treatment trends in pemphigus patients. Compare outcomes to various steroid sparing agents (SSA) and assess corresponding changes in desmoglein antibodies
Methods: Retrospective chart review of pemphigus patients in a single dermatology practice using 17 years of electronic record data. Published clinical outcome endpoints and desmoglein immunoassays were used.
Results: 115 patients PV and PF were reviewed for response to SSA. 88 patients responded to at least one SSA. 60/67 patients had complete remission on minimal therapy with TCN/NAM and 24 of these went on to complete disease remission off all therapy (mean duration off therapy = 48.25 months). Other SSAs included: mycophenolate mofetil (n=31), rituximab (n=18), methotrexate (n=17), azathioprine (n=14), oral gold (n=5), and IVIG (n= 8). Average duration of response was 19-40 months. 43 patients had one flare/remission cycle, 21 had two, and 24 had >3 flare/remission cycles. Desmoglein antibody titers were highly variable during the time to complete remission on therapy.
Limitations: Standardized disease assessment tools could not be applied in this retrospective study.
Conclusion: Long term management of pemphigus patients often requires many therapeutic options and TCN/NAM is effective as an initial SSA in many patients.
Keywords
Pemphigus, Treatment, Tetracycline, Niacinamide, Long term follow up, Steroid Sparing Agents
Citation
Fivenson D, Qiblawi S (2020) Long Term Follow up and Sequential Therapies in Pemphigus: Many Paths to Disease Control and Minimizing Corticosteroid Usage. J Dermatolog Clin Res 8(1): 1128.
ABBREVIATIONS
AIBD: Autoimmune Bullous Disease; DSG-1: Desmoglein 1; DSG-3 - Desmoglein 3; TCN/NAM: Tetracycline and Niacinamide; CDA: Control Disease Activity; CRon: Complete Remission on therapy; CRoff: Complete Remission off therapy; CRmin: Complete Remission on minimal therapy; PV: Pemphigus Vulgaris; PF: Pemphigus Foliaceus; BP: Bullous Pemphigoid; IVIG: Intravenous Immunoglobulin; SSA: Steroid Sparing Agent; ELISA: Enzymelinked Immunosorbent Assay
INTRODUCTION
Pemphigus vulgaris (PV) and Pemphigus foliaceus (PF) are autoimmune blistering diseases (AIBD) of the skin and mucous membranes that usually affect individuals that are 40-60 years old [1]. Blisters and erosions occur in the oral mucosa early on; while more generalized disease can involve any other skin or mucosal surface [1]. Therapy of PV/PF with systemic corticosteroids is used to gain initial control of disease activity (CDA) and promote healing. Once CDA is achieved, a variety of steroid sparing agents (SSA) are used to bring the patient into complete disease remission on therapy (CRon) [1,2]. These include immune modulators like dapsone, intravenous immunoglobulins (IVIG), oral gold, plasmapheresis and tetracyclines combined with niacinamide (TCN/NAM). Systemic immunosuppressive agents including azathioprine, methotrexate, mycophenolate mofetil, and rituximab, are also often used to achieve CRon and limit long term effects of oral steroids [3]. The long-term goal of therapy is complete control and eventual complete remission on minimal or no therapy (CRmin, CRoff), to minimize the side effects of chronic immunosuppressive therapy. The use of tetracycline’s (tetracycline, doxycycline and/or minocycline) plus niacin amide (TCN/NAM) have proven to be a successful steroid sparing treatment regimen in patients with bullous pemphigoid (BP) [3-5] and has also shown to be an effective treatment in PV and pemphigus foliaceus (PF)[6,7]. Rituximab was approved by the FDA in 2018 as a steroid sparing therapy for PV. It’s ability to suppress B-cells producing desmoglein 1 (DSG-1) and desmoglein 3 (DSG-3) autoantibodies has shown to be effective in treating patients with PV and PF, and led to prolonged CRoff [2,7].
While early use of rituximab is becoming more common, many patients have a history of repeated cycles of flaring after periods of CRoff or CRmin. Retreatment with oral corticosteroids and SSAs can occur many times throughout a patient’s lifetime. This chronic disease susceptibility provides for longitudinal follow-up and comparison of different treatment regimens within small patient groups (or even amongst individual patients) over the many decades their disease may afflict them. In 2014, we published a group of PV/PF patients who had good SSA responses to TCN/NAM, and in the present study we review all patients we have treated and compare relative efficacy over time of sequential treatment cycles with TCN/NAM and many of the other SSAs above. We also compare how initiation of these treatment cycles effect titers of DSG-1 and DSG-3.
METHODS
After institutional review board approval, a retrospective chart review was performed of all PV and PF patients in a private medical dermatology office setting, using 17 years of electronic medical record data (2002 - 2019). Patients (n=115) were retrospectively analyzed to determine their response to any SSA therapy. Patients who responded with complete remission on any type of treatment for pemphigus were included in this study (n=88). Data from all newly diagnosed and existing patients that were flaring, along with different SSA responses used to induce remission of these cycles of flare/remission were analyzed. Clinical assessments used a modified version of outcome parameters previously described by Murrell et al. (Table 1) [2,6]. A responder to therapy was defined as CRoff, CRmin, or CRon within 3 months of initiation of all SSAs except rituximab, which was defined as at least 6 months from last infusion due to its prolonged effects on B cell function [8].
Serologic testing
DSG-1 and DSG-3 titers were assessed every 3-6 months in most patients by commercial ELISA (MBL International IgG DSG-1 and DSG-3 antibody ELISA kits) at either Mayo Clinical Laboratories or ARUP Laboratories).
Efficacy assessment
Three months after initiation of SSAs for PV or PF (6 months for rituximab treatment) was chosen as the primary endpoint for retrospective analysis. This was the average amount of time for standard oral steroid induction of CDA, initiation of SSA and steroid tapering by 10% per week [2,9].
RESULTS
There were 88/115 patients treated over the 17 year review period that met the inclusion criteria. The remaining 27 patients were either lost to follow or returned to care of another dermatologist (n=26) or could not be tapered below 20mg prednisone (n= 1). All patients included in the study presented with active disease and were given initial therapy with oral corticosteroids (1-2mg/kg/d) to achieve CDA. SSAs were added at CDA (typically 2-4 weeks after starting steroids) to achieve CRon. SSA treatments varied based on patient severity, but
Table 1: Adapted guidelines for pemphigus definitions from Murrell et al. for clinical assessment of lesions in PV and PF [2].
| Clinical Assessment | Definition |
| Complete remission off therapy | No new lesions for at least 3 months without any therapy |
| Complete remission on therapy | Absence of new or established lesions for at least 3 months with minimal therapy (defined as half of the treatment failure dose) or >/= 10 mg/ day of prednisone |
| Partial remission off therapy | Presence of transient new lesions that heal within one week without treatment and off all systemic therapy for at least 3 months |
| Partial remission on minimal therapy | Transient new lesions that heal within week while on minimal therapy, including topical steroids |
| Flare/relapse | 3 or more new lesions a month that do not heal spontaneously within 1 week, or by the extension of established lesions in patient with controlled disease |
| Minimal Therapy | 10 mg/day prednisone and/or 1 mg/kg/day cyclophosphamide, 10 mg/week methotrexate*, 1.25 mg/kg/day azathioprine, 1.5 gm/day mycophenolate mofetil,* 2 gm/day Tetracycline and 1.5 gm/ day Niacinamide |
| *Based off of a 75kg individual for dosing. | |
generally consisted of starting the patient on a first line trial of TCN/NAM (either doxycycline 100mg twice daily, or minocycline 100mg twice daily with 500mg of niacinamide 3 times daily), and steroid tapering of ~10% per week. Disease flares before tapering to 20mg/d oral steroids were treated with increasing steroids to recapture CDA and switching to another SSA. The most common sequential therapy pattern observed was TCN/ NAM as first line SSA, followed by a sequence of mycophenolate mofetil, then azathioprine, methotrexate, and rituximab. Most of the patients reviewed were treated with immunosuppressives prior to rituximab as the data set extends back to 2002, when this agent was not yet considered standard of care and readily available for AIBD treatment.
TCN/NAM: Of the 67 patients who received TCN/NAM in this study, 60 (89.6%) patients responded to TCN/NAM therapy with durations of 3 months to 9.67 years (mean 26.84 months +/- 24.64 months). Responses (CRmin) ranged from 3 month to 8 years (mean 19 +/- 17.50 months).These 60 patients with CRmin maintained this for an average of 19 months. 24 patients had an average of 48.25 months CRoff after discontinuation of TCN/NAM.
40 patients were controlled on TCN/NAM without any other additional treatment (i.e no combinations with other SSA or low dose oral steroids), and were CRmin, and 24 transitioned to CRoff over a range of 10-51 months. The range of DSG-1 change in patients treated at least 3mo with TCN/NAM is included in figure 1 and was -121 to 36 (mean -3.6 +/- 21.6) and the range of DSG-3 change was -323 to 92 (mean -20.3 +/- 73.38) .
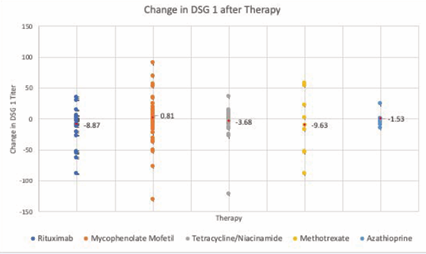
Figure 1 Changes in DSG-1 after each therapy. Scatter plot of all DSG-1 titers collected for each steroid sparing agent from all patient data points available. The red data point noted in each therapy represents the mean change in DSG-1.
SSA Responses
Azathioprine: Of the 88 patients who responded to SSAs, all 14 patients treated with azathioprine achieved CRon. The change in DSG-1 was -15 to 24 (mean -1.53 +/- 8.41) and DSG-3 changes ranged from -38 to 36 (mean -5.86 +/- 17.2).
Methotrexate: Of the 88 patients who responded to SSAs, all 17 patients treated with methotrexate achieved CRon. The range of changes in DSG-1 were -88 to 57 (mean -9.63 +/- 53), and changes in DSG-3 ranged from -86 to 73 (mean -6.38 +/- 48.9).
Mycophenolate Mofetil: Of the 88 patients who responded to SSAs, 31 patients treated with mycophenolate mofetil achieved CRon, 5 patients did not achieve CRon (still requiring >20mg/d oral steroids) while on mycophenolate mofetil. Changes in DSG-1 ranged from -130 to 90 (mean 0.814 +/- 33.88), and changes in DSG-3 ranged from -246 to 90 (mean -13.56 +/- 52.79).
Rituximab: Of the 88 patients who responded to SSAs, 18 patients treated with rituximab achieved CRmin, 6 months after infusion and 3 patients did not achieve CRmin (still requiring >20mg/d oral steroids) after 6 months of being treated with rituximab. Changes in DSG-1 ranged from -89 to 35 (mean -10.42 +/- 28.15) and changes in DSG- 3 ranged from -515 to 129 (mean -32.69 +/- 42.19).
Gold: Of the 88 patients who responded to SSAs, all 5 patients treated with gold therapy (oral auranofin) achieved CRmin. There were minimal changes in DSG-1 and/or DSG-3, but all 5 patients achieved the primary end point of CRmin.
IVIG: Of the 88 patients who responded to SSAs, 8 patients treated with IVIG therapy achieved CRmin, 3 patients did not achieve Crmin (still requiring >20mg/d oral steroids). DSG-1 and DSG-3 levels for the patients that were placed on IVIG were not drawn.
Duration of therapeutic responses
The mean duration of response with these SSAs was 26.25(range 6-48) months for azathioprine, 35.5 (range 10-103) months for methotrexate, 27.38 (range 3-80) months for mycophenolate mofetil, 32.2 (range 3-46) months for rituximab, 39.75 (range 34-56) months for gold, and 22.5 (range 6 to 52) months for IVIG.
Multiple/Sequential Treatment Cycles
43 patients had one flare to remission cycle, 21 had two flare to remission cycles and 24 had three or more flares with periods of remission (ranging from 3 to 87 months).
DISCUSSION
The management of pemphigus can be challenging and often times requires trials of multiple immunosuppressive therapies to achieve CRon and ultimately CRoff for patients. The aim of this study, and treatment in general, is to find a treatment that reduces disease burden while minimizing medication-related toxicities.
Given the nature of this retrospective analysis, it was difficult to clarify long-term outcomes in many patients. 60 patients were clearly documented as CRmin for an average of 19 months, and 24 patients had an average of 48.25 months follow up after discontinuation of TCN/NAM. The mechanism of action of TCN/ NAM in AIBDs is unknown but may be related to suppression of leukocyte migration [3-6].TCN/NAM was overall similar to the other SSAs which had mean CRmin/CRoff durations of therapy averaging from 22.5 months to 39.75 months. Thus TCN/NAM may be considered as a first line SSA, on par with other agents in efficacy for initial CR, but of minimal immunosuppressive risk compared to other SSAs. Therefore, flares of pemphigus were seen in most patients after 2-4 years of treatment.
The relationship between changes of desmoglein titers in our patients did not seem to have a distinct pattern and we noted an increase in DSG-1 and DSG-3 titers at CRon/CRmin in many patients, irregardless of SSA used (figures 1 and 2).
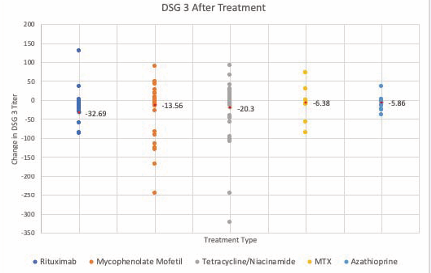
Figure 2 Changes in DSG-3 after each therapy. Scatter plot for all DSG-3 titers collected for each steroid sparing agent from all patient data points available. The red data point noted in each therapy represents the mean change in DSG-3.
Short term fluctuations of pemphigus titers during initial therapy have been noted in other studies and that the persistently high DSG titers do not necessarily equate to disease activity, but rather it is the change in titers that show disease flaring [10,11]. While some patients did increase titers after achieving CRon, the average response to treatment showed a drop in both DSG 1 and DSG 3 titers. The exception was there was no net change in DSG-1 titers in mycophenolate mofetil responders (mean change 0.814 +/- 33.88). Longer followup of responders would have been expected to demonstrate DSG1 and DSG3 titers trending lower as previously reported [10-14]
Nevertheless, we saw that there was a clinical remission at the 3 month primary endpoint in patients started on SSAs, regardless of how high DSG-1 and DSG-3 titers were. The overall average change in each titer was indicative of disease control, but there was wide variation (figures 1 and 2) amongst individuals. Weiss et al. and Russo et al., also noted clinical improvement of PV is not always correlated with a decrease in DSG antibodies [10,11]. Our findings are consistent with other studies showing that initial clinical responses may be accompanied by persistently high titers that come done over time [11,12,15]. Our observations of increased DSG titers may be related to our 3mo primary endpoint, as this may have been before the majority of antibody producing plasma cells were cleared from the circulation. Similar 3 month initial response times in studies with rituximab also cite this as a time point for when the maturation process of pre-B cells into mature B cells and ultimately plasma cells would reach peak effects. Prolonged cycling of Ig via the FcRn receptor might explain the persistence of high titers despite clinical responses in and around this 3 month time point [15-18]. An ongoing phase III clinical trial (NCT03762265) with a Burton tyrosine kinase inhibitor which interrupts this recycling of IgG may answer this question as preformed IgG has a much shorter half-life in the circulation in the presence of this inhibitor [19].
The average patient seen in our practice received treatment for 3.83 years before being taken off of therapy (CRoff) and was placed on an average of 1.9 therapies during that time. The most common steroid sparing treatment started after steroid therapy was TCN/NAM (n= 67) which resulted in CRmin at 3 months in 89.6% of patients. Rituximab was used in persistent disease that was not responding to other immunomodulators/ immunosuppressants commonly used in pemphigus (i.e. azathioprine, mycophenolate mofetil, methotrexate, gold, IVIG and dapsone). Rituximab showed the largest change in DSG-3 titers (mean -32.69 +/- 42.19). Increases in DSG1 and DSG3 were also seen in some of these patients as well (Figures 1,2).
LIMITATIONS
Standardized disease assessments (e.g PDAI or ABSIS) were not used in this retrospective, single practice study, therefore ‘gestalt’ response benchmarks were used based on abstracted clinical data. The clinical response at 3 months was used partially to compensate for the descriptive nature of the clinical data.
CONCLUSION
We have reviewed clinical data over 17 years from 115 pemphigus patients and show support our prior publication on the efficacy of TCN/NAM as a first line SSA. We also have shown that the average durability of TCN/NAM response is 19 months. The pattern of second and third line SSAs in resistant patients in our practice is typical of other treatment reviews [20]. We have expanded these observations and found initial clinical responses did not always parallel decreases in DSG-1 or DSG-3 titers in the first 3-6 months after initiation of treatment. We did see that rituximab was associated with the largest average change in DSG-3 titers of any SSA, similar to other reports [8], yet individual responders had net increases as well. Much longer term serologic follow-up may be needed to see the expected downward trends in titers after CR. TCN/NAM may be an effective monotherapy for disease control in pemphigus, while rituximab can have robust effects on patients that are relapsing or have poor initial disease control.









































































































































