Safety and Cost Effectiveness of a Single Outpatient Encounter for Initiation of Propranolol in Treatment of Infantile Hemangioma
- 1. Department of Pediatrics, UPMC Children’s Hospital of Pittsburgh, USA
- 2. Paul C. Gaffney Division of Pediatric Hospital Medicine, UPMC Children’s Hospital of Pittsburgh, USA
Abstract
Objective: There remains variability in initiation protocols for propranolol in patients with infantile hemangiomas including rapid inpatient titrations and slow outpatient protocols. The aim of this study was to determine the utility of a 2-hour outpatient visit to initiate propranolol treatment.
Methods: The outcome measures of utility were complication rates (hypoglycemia, bradycardia, hypotension) and overall cost. 166 patients were included over a 5-year period. All patients were initiated at goal dose of propranolol (2 mg/kg/day divided twice daily). Three prospective cohorts were compared: 48-hr inpatient titration (0.5 mg/kg/dose for 2 doses then 1 mg/kg/dose for 2 doses), 24-hr observation admission (1 mg/kg/dose for 2 doses), and a 2-hour outpatient visit (1 mg/kg/dose for 1 dose). All patients received a screening EKG and hypoglycemia teaching. Patients were excluded for expedited PHACES evaluation, subglottic stenosis, EKG with conduction delay, or if already admitted at time of diagnosis. Gestational age and weight were not determining factors.
Results: There were zero episodes of hypoglycemia, hypotension or bradycardia during initiation or maintenance phase for all cohorts. Cost was dramatically different between the cohorts; controllable expenses were reduced by 1000%. The average cost for the 48-hour cohort was $3,521 versus $350 for the 2-hour outpatient cohort. The total cost savings in the first year of the 2-hour outpatient initiation cohort was over $200,000.
Conclusion: Outpatient initiation of propranolol with a single dose at goal (1 mg/kg/dose twice/day) and a 2-hour observation period is safe and cost effective.
Citation
Ghuman A, Marathe P, Tarchichi T, McCormick A (2020) Safety and Cost Effectiveness of a Single Outpatient Encounter for Initiation of Propranolol in Treatment of Infantile Hemangioma. J Dermatolog Clin Res 8(1): 1129.
ABBREVIATIONS
IH: Infantile Hemangioma; CHP: Children’s Hospital of Pittsburgh; PHACES: Posterior Fossa, Hemangioma, Arterial Lesions, Cardiac Abnormalities, Eye Abnormalities: EKG: Electrocardiogram
INTRODUCTION
Infantile hemangiomas (IH) are vascular lesions that are one of the most common benign vascular tumors of infancy and childhood with an incidence of 3 to 10% [1]. The natural history of IH includes a growth phase followed by an involution phase; this contrasts with other vascular malformations which generally grow and do not regress. The duration and rate of growth of IH lesions are variable; some infants have hemangiomas with minimal growth, while others’ grow rapidly and at an unpredictable rate [2]. The majority of growth occurs during the first five months after diagnosis. Most lesions follow an uncomplicated course, but approximately 12% result in complications requiring referral to a specialist, which include ulceration, bleeding, and obstruction or compromise of underlying organ function based on location of the hemangioma (e.g., airway or GI tract) [3].
Therapy for IH has evolved over time, beginning with systemic steroids and later vincristine and interferon alpha. Since 2008, propranolol has proven to be a more effective treatment of IH than systemic steroids. It also has a greatly increased safety profile. The most serious adverse effects related to propranolol are bradycardia, hypotension, and hypoglycemia. Other reported side effects include bronchospasm, somnolence, sleep disturbance, nightmares, gastroesophageal reflux, nausea, vomiting, and diarrhea [4]. In 2011, the American Academy of Pediatrics held a consensus conference regarding the use of propranolol as treatment of IH in pediatric patients. The committee acknowledged that there was a lack of high-quality clinical research data that would allow for evidence-based recommendations on initiation, dosing, and safety profiles of propranolol treatment. However, the committee did agree upon a set of recommendations regarding this treatment, including obtaining pre-treatment EKGs and monitoring heart rate and blood pressure 1-3 hours after administration as this is when the effect of propranolol is the greatest [2].
Currently, the standard of care for IH is treatment with propranolol dosed at 2-3 mg/kg/day divided twice a day or three times a day for 6 months or until the patient turns 1 year of age. The consensus guidelines presently recommend one of two possible initiation protocols. The first protocol is to rapidly titrate propranolol in the inpatient setting over a course of 48 hours while monitoring for side effects. This approach is currently utilized for children < 48 weeks gestationally corrected age or with comorbid conditions. The second approach is to begin therapy at 1 mg/kg/day of propranolol and slowly up-titrate by 0.5 mg/kg/day every three to seven days in the outpatient setting to goal of 2 mg/kg/day. This method is utilized in older children (> 48 weeks gestationally corrected age) and with good social support [2].
Prior to this study, our approach at the Children’s Hospital of Pittsburgh (CHP) was to rapidly titrate propranolol over a 48-hour hospitalization with close observation for side effects. Initial dosing of propranolol began at 1 mg/kg divided three times a day for 24 hours, and then increased to the goal dose of 2 mg/kg divided three times a day for 24 hours.
The literature showed that less than 1% of children had clinical bradycardia, hypotension, or hypoglycemia with treatment [5]. Given the low rate of adverse side effects, it was thought that initiation could take place over an abbreviated time course. A 2-hour initiation protocol was developed based on the pharmacodynamics of propranolol as it reaches peak effect on heart rate and blood pressure approximately 2 hours after administration [2].
There remains wide variability in initiation protocols for propranolol treatment for IH including rapid inpatient titrations and slow outpatient titrations as the two consensus models. The aim of this study was to determine the safety and cost efficacy of a 2-hour outpatient visit to initiate propranolol at goal (2 mg/ kg/day divided twice per day) with a single dose of 1 mg/kg for treatment of IH.
PATIENTS AND METHODS
IRB approval was obtained prior to the start of this study. 166 patients with infantile hemangiomas were included in this study over a five-year period. There was no prior standard of care for hospitalization or admission status and thus cohorts were determined based on the admitting clinician’s judgement. Three prospective cohorts were compared: a 48-hour inpatient drug titration (0.5 mg/kg/dose x 2 doses, then 1 mg/kg/dose x 2 doses), a 24-hour observation admission (1 mg/kg/dose x 2 doses), and finally a 2-hour outpatient initiation visit (1 mg/kg/dose x 1 dose) (Figure 1).
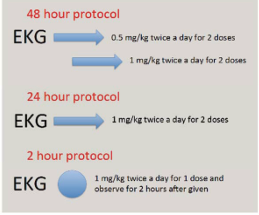
Figure 1 Comparison of different protocols for propranolol initiation.
There were 56 patients in the 48-hour cohort, 51 patients in the 24-hour cohort, and 59 patients in the 2-hour outpatient cohort. Patients in all cohorts received a screening EKG, pre-prandial glucose measurements, continuous cardiac monitoring, and home metered blood glucose (MBG) teaching. Exclusion criteria included patients with need for expedited PHACES evaluation, critical subglottic stenosis, EKG with conduction delay, or if they were already admitted at the time of diagnosis. Gestational age and weight at initiation of therapy were not determining factors. Complication rates (hypoglycemia, bradycardia and hypotension) were monitored to assess safety. Blood glucoses were checked before every feeding, or about every 3 hours. Heart rate was monitored continuously and blood pressures were measured and documented every 4 hours. Normal ranges as stated in the AAP consensus conference paper were used for bradycardia: heart rate less than 70 for newborns less than 1 month old, less than 80 for infants 1-12 months old, and less than 70 for children more than 12 months old. Hypoglycemia was defined as blood glucose < 60 for all patients. The primary outcome measure was overall cost.
RESULTS
There were zero episodes of hypoglycemia, hypotension, or bradycardia during the initiation or maintenance phases of treatment in all three cohorts (Figure 2).
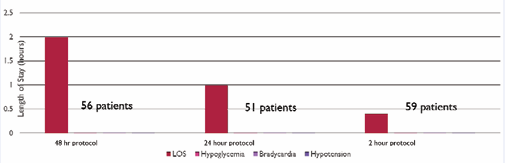
Figure 2 Comparison of safety events across different protocols.
Reported side effects during propranolol therapy did include one patient with sleep disturbances, one patient with gastroesophageal reflux and diarrhea, and one patient with sleep disturbances and gastroesophageal reflux.
There was a dramatic difference in cost among the three cohorts. The average total expense for the 48-hour drug titration cohort was $3,521. The average total expense for the 24-hour drug titration cohort was $1,086. The average total expense for the 2-hour outpatient visit cohort was $350. The total costs saved in the first year of the 2-hour outpatient initiation visit cohort were $212,341. The controllable expenses were reduced by 1,000% with the 2-hour outpatient initiation visit cohort (Figure 3).
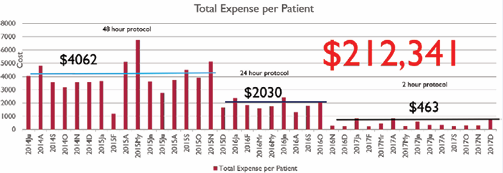
Figure 3 Comparison of cost per patient across different protocols and total cost savings of 2-hour protocol in comparison to 48-hour protocol.
DISCUSSION
This study demonstrates that rapid initiation of propranolol during a brief 2 hour outpatient encounter is a safe and costeffective method of beginning treatment for IH.
The cost savings data is reflected by a cost analysis study by Chaturvedi et al., which showed a theoretical $2,465 expected cost difference between a one-day hospitalization stay for propranolol initiation vs. outpatient initiation [1]. This study applies this cost analysis approach to demonstrate real-world savings of 1000% and over $200,000 per year for the healthcare system. Beyond basic cost savings analysis, there can be argued a significant value to families. Families incur both time and financial cost with multiple outpatient visits and with a prolonged inpatient hospitalization. The ability to streamline initiation brings value not only to the health care system, but importantly also to families.
The findings regarding the safety of propranolol initiation in this study build on the findings of prior slower outpatient up titration studies as shown by Fogel et.al., who showed no adverse side effects requiring clinical intervention in a three-day propranolol uptitration protocol [6]. This is also shown in other outpatient initiation studies [7-9]. Propranolol has been long shown to have an excellent safety profile and this study confirms that safety can be maintained in a brief outpatient initiation visit.
at safety can be maintained in a brief outpatient initiation visit. Importantly, this data argues against the current recommended consensus guidelines for initiation of propranolol. First, gestational age was not a determining factor in our study and there were no side effects observed for any age, even younger infants. Therefore, we argue against admitting infants to the hospital based solely on gestational age less than 48 weeks. Second, we initiated propranolol at goal dosing (single dose of 1 mg/kg) rather than using a multi-step uptitration protocol with multiple outpatient visits as stated in the consensus guidelines [2]. Again, there were no major adverse side effects of bradycardia, hypotension or hypoglycemia observed.
The main limitation of this study is that side effects were based on parent report and chart review of emergency room visits.
CONCLUSION
Propranolol is the gold standard for treatment of IH and is both effective and safe to initiate over a 2-hour observation period. Outpatient initiation of propranolol should be done for infants and children of all ages who do not have PHACES, subglottic stenosis, or conduction delay on EKG. At our hospital, we have been able to establish and implement clinical effectiveness guidelines and partner with our observation unit for initiation of propranolol during a 2-hour observation stay. Parents are sent home with feeding instructions and guidelines on when to hold propranolol, when to check MBGs, and when to follow-up. There are also significant cost savings with this expedited treatment protocol. Our data adds to the 2011 consensus conference statement in that a single outpatient initiation visit with a 2-hour observation period after one dose of propranolol at 1 mg/kg/dose two times a day is safe and cost-effective for all ages. Our method can be implemented across hospitals if there is a short stay observation unit available and if there are educators available to do home teaching.









































































































































