The Effect of Ceramide Analogues on Normal Skin and Hypertrophic Scar Fibroblasts
- 1. Department of Plastic and Reconstructive Surgery, Hadassah Medical Center, Israel
- 2. Department of Plastic and Reconstructive Surgery, Hadassah Medical Center, Israel
- 3. Department of biochemistry, The Hebrew University, Israel
Abstract
Background: Fibroblasts play a key role the in skin healing process, in both normal and abnormal scarring. Excessive scarring is believed to be related to lack of normal apoptosis of skin fibroblasts. Ceramide has a known effect on intra-cellular signaling and apoptosis.
Aim of the study: To examine the effect of synthetic ceramide analogues on normal skin fibroblasts and on fibroblasts from hypertrophic and keloid scars.
Methods: Skin fibroblasts were cultured from biopsies of normal skin donors. Cell cultures were then incubated with two synthetic ceramide analogues to determine the LD50 of the ceramide analogue. Ceramide analogues were added to fibroblasts cultures derived from hypertrophic and keloid scar tissue samples. These were examined for cell growth and for pro-apoptotic proteins levels.
Results: The two synthetic ceramide analogues tested were found to significantly inhibit cell growth in fibroblast cell cultures. The effect of ceramide analogues was more noticeable on cultures from hypertrophic and keloid scars than on cultures from normal skin. Ceramide analogues also caused a proapoptotic change in protein level expression.
Conclusion: Ceramide analogues have a pro-apoptotic effect on fibroblasts, which is more profound in excessive scar tissue compared to normal skin. This may be useful information when treating hypertrophic or keloid scars.
Citation
Shachar Y, Meir R, Ben-Yehuda G, Zaga J, Chaouat M, et al. (2018) The Effect of Ceramide Analogues on Normal Skin and Hypertrophic Scar Fibroblasts. J Dermatolog Clin Res 6(2): 1118.
INTRODUCTION
Excessive scarring may cause body disfigurement and function loss, as well as a profound psychological effect. Although many prophylactic and therapeutic strategies are available for hypertrophic scarring, no single therapeutic modality is universally effective. Recurrence rates with excision alone are 45-100% and remain high even with aggressive treatment. Differences between keloids, hypertrophic scars and normal scars include distinct scar appearance, histologic morphology and cellular function in response to growth factors. The cellular mechanism of excessive scar formation is complex and not fully understood. Lack of a balanced apoptosis mechanism is believed to be fundamental in hypertrophic scar formation. In the absence of animal models, investigation of excessive scarring in-vivo is very limited.
Ceramide is a sphingolipid known to induce apoptosis in normal fibroblasts. The influence of ceramide on hypertrophic scars, however, is less well understood. TGFβ is over expressed in hypertrophic scars and its secretion by fibroblasts is inhibited by ceramide. Fibroblasts were shown to react differently to ceramide in several fibrotic conditions. Fibroblasts in keloids are thought to be resistant to ceramide induced apoptosis. This was shown to be related to differences in the Fas receptor pathway [1-11].
The first stage of our research aimed to analyze the effect of two ceramide synthetic analogues on normal skin fibroblasts in growth inhibition and their effect on some main signal transduction elements. We then examined the effect on hypertrophic scar fibroblasts, comparing the results to those of normal fibroblasts.
METHODS
The study was approved by the medical and ethical committees of our hospital, and patients signed an informed consent before the study.
Fibroblast growth
1 cm sq. split thickness skin samples were taken from normal skin of five patients and from excessive scar tissues of five patients (4 hypertrophic scars and one keloid). All samples were inspected under light microscopy for confirmation of clinical diagnosis. The skin was washed with phosphate buffer saline, minced to 1mm pieces and transferred in to cell culture medium containing DMEM (Dulbeco Modified Eagle Medium with D-glucose 4500 mg/L without glutamine and pyruvate, Biological Industries Ltd, Beit Haemek, Israel) with 10% FCS (Fetal Calf Serum, Charcoal Stripped, Biological Industries Ltd, Beit Haemek, Israel), Glutamine and antimicrobial solution (Penicillin, Streptomycin, Amphotericin B, Biological Industries Ltd, Beit Haemek, Israel). The cell cultures were propagated and incubated at 37°C with 5% CO2 . Fibroblasts were then seeded in a 96 wells plate with either 5000 or 10,000 cells in each plate, with 200λ of medium. Two synthetic ceramide analogues (2750, 2900) were added to the wells 24 hours after seeding, in descending concentrations. At days 0,1,3,5,7, plates were fixated with 50λ of 2.5% glutyraldehyde (Sigma Israel Chemicals Ltd.) for twenty minutes and then washed. Plates were then colored with Methylen blue 1% (Reidel – de Haen laboratory chemicals, Hanover, Germany), rinsed and dried. Pigment was extracted with 200λ of HCl and then incubated for 60 minutes. Optical density was measured at wave length of 620nm (Anthos II Microplate Reader, Viena Austria).
Western blot electrophoresis was performed with rabbit anti-Bcl2, anti-Bax and anti-TGFβ (Cell Signaling Technology, Danvers, MA, USA).
Statistical analysis
One way ANOVA test was used for each ceramide analogue concentration. When results were significant, data was examined using Post HOC test, according to Dunnett3 approach. KruskalWallis test was used for a-parametric variance analysis.
RESULTS
Growth curves: The addition of each of the ceramide analogues caused an arrest of fibroblast proliferation. This was dose dependent, as can be seen in Figure (1).
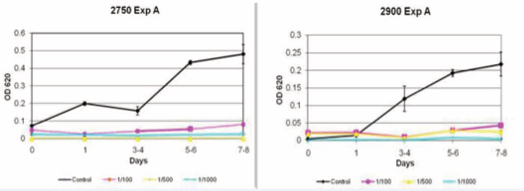
Figure 1 Inhibition of fibroblast with ceramide analogue 2750 (left) and 2900 (right). *OD= Optic Density.
These results were accurate for both normal skin and scar fibroblasts. The difference was significant (p<0.0001) for both groups and was more pronounce for the 2900 analogue (Figure 2).
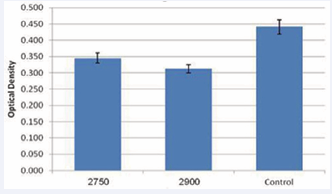
Figure 2 Optic density of fibroblasts after administration of synthetic ceramide analogues 2750 and 2900 (p<0.0001)
The differences were apparent 24 hours after exposure and increased with time. The effect of ceramide analogues was more significant on hypertrophic scar tissue fibroblasts than on normal skin (p<0.007, Figure 3).

Figure 3 Effect of ceramide analogue on optic density of normal and scar tissue fibroblasts in a 24hr period. (p<0.007).
here was no statistical significant difference in the levels of the proteins Bcl2, Bax and TGFβ between the ceramide analogues and control groups (Figure 4).
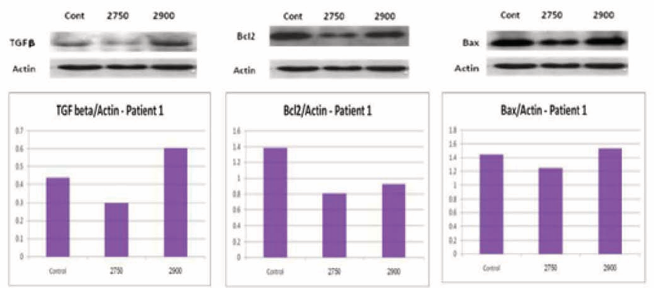
Figure 4 TGFβ, Bcl2 and BAX levels in normal fibroblasts with and without ceramide analogous.
In normal skin fibroblasts, the ratio of Bax/Bcl2 was 1.2 in the control group comparing to 1.41 in the 2750 analogue and 1.64 in the 2900 analogue groups. This trend toward a pro-apoptotic cell signaling was not statistically significant. The same trend was noticed when repeating the test with fibroblasts from scar tissues. When comparing fibroblasts from scar tissue and normal skin, the former group showed lower levels of BAX/Bcl2, regardless of ceramide treatment. This was not statistically significant, but correlates with lower proapoptotic protein levels in scar tissues comparing to normal skin.
DISCUSSION
Hypertrophic scars and keloids cause a significant discomfort to many patients, despite vigorous research and multitude of treatment modalities. Although many prophylactic and therapeutic strategies are available for hypertrophic scarring, no single therapeutic modality is universally effective. Why some patients develop unsightly and sometime debilitating scars while others heal in a more favorable manner is not completely clear. In our preliminary research, we tried to characterize the difference in intracellular cell signaling between normal skin and scar tissue fibroblasts. Other authors were able to demonstrate changes in apoptosis related protein levels in reaction to ceramide. We were not able to demonstrate a statistical difference in the levels of the proteins Bcl2, Bax and TGFβ in our research, probably due to the small amount of samples. We did, however, show a trend toward a less pro-apoptotic protein profile in hypertrophic scar fibroblasts then in normal skin fibroblasts, as shown by the BAX/ Bcl2 ratio.
Ceramide analogues were found to be very potent at causing cell arrest. Moreover, the effect of ceramide was greater when applied to cultures of scar fibroblasts comparing to normal skin. This may be very important when considering development of future treatments for hypertrophic scarring. Our research mainly examined the effect of ceramide on hypertrophic scars. We did include one sample from a keloid scar in our study group. The diagnosis of keloid in this specimen was confirmed histologically by light microscopy. In this sample, we observed a very subtle change after the addition of ceramide analogues. This finding may be supported by previous study (Ishihara et al), presenting evidence of keloid resistance to ceramide-induced apoptosis.
Additional studies based on a larger sample size of both normal and scar tissue fibroblasts specimens are needed to further explore the effect of ceramide and other sphingolipids. Based on the promising results shown above, we believe that scars could be selectively targeted and effectively treated.
CONCLUSION
In this study we can assess how ceramide analogous have a pro-apoptotic effect on fibroblasts, which is more profound in excessive scar tissue comparing to normal skin. Despite the small sample of the study consider that ceramide analogous may become useful when treating hypertrophic or keloid scars.
PRODUCTS
• Biological Indusrties Ltd (Beit Haemek, Israel): DMEM – Dulbeco Modified Eagle Medhium (with D-glucose 4500 mg/L without glutamine and pyruvate.) cat #: 01-055-1A Foetal Calf Serum (cFCS) cat #: 04-201-1B Charcoal Stripped Penicillin, Streptomycin, Amphotericin B cat #: 03-033-1B Trypsyn-EDTA solution B # cat: 03-052-1A
• Sigma Israel Chemicails Ltd: Monoclonal anti β-actin cat #: A5441 Glutaraldehyde 8% cat #: G7526
• Teva Pharmceuticals Ltd, Israel: Gentamicin
• Merck Serono Ltd. Israel: Boric acid (H3 BO3 )
• Bio-Rad Laboratories, Israel: Lyphilized Bovine Plasme Gamma Globulin, cat #: 500-0005 Acrylamide 40%, cat #: 161-0148
• Amresco, Solon ,Ohio, USA: Albumin Bovine (PH 7.0), cat #: 0332-50G
• Cell signaling technology Inc., Danvers, MA, USA: Anti Bcl-2 – Rabbit 2875 Anti Bax – Rabbit 2872 Anti TGF-β−Rabbit 3712
• Amersham International plc, Buckinghamshire, England: Rainbow colored protein molecular weight marker cat #:56-81-5
• Jackson Immuno-research Ltd. Suffolk, UK: Goat anti mouse IgG (H+L) peroxidase – conjugated. cat #: 115- 035-003
• Riedel - de Haen, Hanover, Germany: Methylene Blue cat #: 28514
REFERENCES
6. Shea BS, Tager AM. Sphingolipid regulation of tissue fibrosis. Open Rheumatol J. 2012; 6: 123-129.
11.Zhang T, Saghatelian A. Emerging roles of lipids in BCL-2 familyregulated apoptosis. Biochim Biophys Acta. 2013; 1831: 1542-1554.









































































































































