Altered Functional T Cell Subset Populations and Cytokine Profile in Patients with Chronic Fatigue Syndrome: A Pilot Study
- 1. Department of Immunology, St. Helier University Hospital NHS Trust, UK
Abstract
Chronic Fatigue Syndrome (CFS) is characterized by disabling fatigue with a delayed post-exertional malaise, headaches, sleep disturbance, arthralgia and myalgia. We have shown several B cell abnormalities in patients with CFS and speculated on an underlying subtle humoral immune dysfunction. As B cells are regulated by certain T cell subsets and the associated cytokines we considered whether an underlying T cell dysfunction may be contributory.
We therefore characterized the different T-cell subsets, T regulatory cells and NK cells and the serum levels of those cytokines involved in regulating different components of the immune system in 32 patients with CFS fulfilling the Canadian and Fukada criteria for CFS. The results were compared with 24 age and sex matched healthy controls (HC).
CFS patients had significantly increased serum levels of interleukin (IL) 12, 21, 22, 27 and TNF-α. We also found CFS patients had significantly greater numbers of T helper memory effector cells, T helper effector cells and cytotoxicity effector T-cells. Patients also had significantly reduced numbers of CD8+ lymph node homing naïve and memory T-cells. T regulatory cells, Th1/Th2 intracellular cytokines, NK cell numbers and NK cytotoxicity were not significantly different between the patient and control groups.
These results confirm that CFS patients have alterations in T cell subsets and cytokines that are involved in regulating cellular immune responses and B cell function. While the cause of these findings is unclear, we discuss their relevance in the context of a possible underlying subtle autoimmunity.
Keywords
• Naive and memory T cells
• Cytokines
• Chronic fatigue syndrome
• Autoimmunity
Citation
Ford B, Bradley AS, Bansal AS (2016) Altered Functional T Cell Subset Populations and Cytokine Profile in Patients with Chronic Fatigue Syndrome: A Pilot Study. J Chronic Dis Manag 1(1): 1004.
ABBREVIATIONS
CFS: Chronic Fatigue Syndrome; ME: Myalgic Encephalomyelitis; NK Cells: Natural Killer Cells; HC: Healthy Controls; IL: Interleukin; CD: Cluster of Differentiation; CCR7: C-C Chemokine 7; TNF-Α: Tumor Necrosis Factor Alpha; IFN: Interferon Gamma; PCP: Peridin Chlorophyll A-Protein; APC: Allophycocyanin; FITC: Fluorescein Isothiocyanate; PE: Phycoerythrin; PBS: Phosphate Buffered Saline; BSA: Bovine Serum Albumin; PBMC: Peripheral Blood Mononuclear Cells; CO2.: Carbon Dioxide; DIOC: 3,3’-Dihexyloxocarbocyanine Iodide; Ig: Immunoglobulin; Th1: T Helper 1; Th2: T Helper 2; Th17: T Helper 17; TCM: T Central Memory Cell; TEM: T Effector Memory Cell; TFH: T Follicular Helper Cell; Treg: T Regulatory Cell; EBV: Epstein Barr Virus; Dutpase: Deoxyuridine-Triphosphatase; STAT3: Signal Transducer and Activator of Transcription 3; Pdc: Plasmacytoid Dendritic Cells; ESR: Erythrocyte Sedimentation Rate; CRP: C Reactive Protein; NKSF: Natural Killer Cell Stimulating Factor; Da: Dalton; BZLF1; Bamhi Z Fragment Leftward Open Reading Frame 1; PMA; Polymyristate Acetate
INTRODUCTION
Chronic Fatigue Syndrome (CFS) is a variably incapacitating heterogeneous disorder of unknown cause but with a common set of symptoms. It is diagnosed on the basis of patients fulfilling one or more of several criteria [1-4] and in the absence of an alternative medical or psychiatric cause of the fatigue. There are various estimates of the prevalence of CFS [5-7] but it is likely to lie between 0.2-0.5%. Unfortunately there are no diagnostic laboratory tests that can diagnose CFS. However, the diagnosis requires that patients return essentially normal results on routine tests of hematological, biochemical, endocrine and immunological function as well as investigations checking for inflammation and gluten sensitivity.
We have observed increased numbers of naive B cells, transitional B cells and plasma blasts in patients with moderate CFS without depression and anxiety who were selected on the basis that they fulfilled the Fukada, Oxford and Canadian criteria [8]. Moreover, the finding of auto antibodies in patients with CFS [9,10] also suggests B cell dys-regulation and has raised the possibility of CFS having an autoimmune component. This is further supported by the recent work indicating clinical efficacy of rituximab, an anti-CD20 monoclonal antibody, in CFS [11].
B cells require T cell help for normal development and function. We therefore sought to investigate alterations in T cell populations and cytokine dys-regulation as factors that may contribute to possible abnormalities of B-cell development in CFS patients. Additionally, many patients with CFS mention frequent sore throats with tenderness of the neck lymph glands and a predisposition to recurrent viral type symptoms. Clinically they appear to manifest a subtle impairment of cellular immunity and T cell dysfunction. A recent study also examined B cell and T cell sub sets in 22 CFS patients compared to 30 healthy controls [12]. This group found no alterations in B cell subset or proliferation; however they used the Fukuda criteria alone to diagnose CFS that may explain the differences between this study and our previous study [8,12]. The study did, however, find significant differences in the NK and T cell subsets with CFS patients having reduced numbers of NK cells (as a % of lymphocytes) and reduced CD8+ T cells (as a % of T cells) [12]. More specifically, the T cells appeared to be generally hypo-responsive with lower levels of effector CD8+ T cells (CD45RA- CCR7- CD27- CD28+) and increased levels of T regulatory cells defined by CD4+ CD25++ FOXP3+ [12].
Several cytokines are involved in regulating the cellular immune system that controls viral infections. CFS patients also appear unable to optimally recover from viral infections suggesting possible deficiencies. Many patients report recurrent sore throats with cervical lymphadenopathy that happens prior to a relapse. Previous studies have reported discordant results on the cytokine profiles of CFS patients. However, several have found patients with CFS to have significantly increased IL10, IFN-γ and TNF-α levels indicating a TH1 bias [13-15]. More recently, Wyller et al. [16], found no difference in 27 plasma cytokines, chemokines and growth factors between 120 adolescent patients fulfilling a ‘broad’ Fukada criteria for CFS and 68 age and sex matched healthy controls. In contrast Nu et al. [17], investigating 16 ‘pure’ CFS patients without objective sleep disturbance found increased levels of IL1, IL8, IL10 and TNF-α. Thus patient selection appears very important in elucidating subtle immune abnormalities in those with CFS.
MATERIALS AND METHODS
Patients and controls
The CFS patient and healthy control cohorts that were used in our study examining B cell populations in patients with CFS were used in this study (Bradley, Ford & Bansal 2013). All participants gave informed consent to the protocol approved by the Central London REC 1 Research Ethics Committee (Rec No: 09/ H0718/54). All patients fulfilled the Canadian and International Consensus Criteria as well as the more recent suggestion by the Institute of Medicine. Importantly all the patients scored 10 or more out of 13 on the Bansal scoring system for CFS [18] and patients had no evidence of significant depression or anxiety. Tests for inflammation, organ dysfunction, endocrinopathy, autoimmunity and gluten sensitivity were negative. Samples collected for the previous study were used in parallel for this study, for details of selection and consent see [8]. Volunteer hospital staff provided the age and sex matched healthy controls and were from the same general geographical area as the patients. Twenty four healthy controls and 33 CFS patient samples were tested, 1 patient’s sample was only sufficient for T (and B) immunopheno typing analysis and therefore was not tested for cytokine levels or the other cellular tests. Four other patients in all were not tested for T regulatory cells and CD17 T cells as a result of a flow cytometer problem. This did not alter the demographics significantly.
Cytokine measurement
Serum was separated from venous samples by centrifugation at 2500.g for 10 minutes within 2 hours of collection and stored at -80°C for batch analysis. Cytokines were measured on a Biorad Bioplex 200 platform using Millipore reagents following manufacturer’s instructions and the data analysed using Biorad Bioplex manager 4.1.1 software. Cytokines representing a broad range of immune function were assayed. These included IFNγ, IL 10, IL 12 p70, IL 15, IL 22, IL 2, IL 21, IL 6, IL 27 andTNF-α.
T cell phenotyping
Briefly 1 mL of whole blood (EDTA) was washed three times in PBS/Azide at (600g for 90 seconds). The blood was re-suspended in 1mL of PBS/Azide/BSA. Five tubes per patient were appropriately labelled and antibody combinations were added as in (Table 1).
Table 1: Antibody Combinations for T cell Phenotyping.
| Tube | Antibody panels | |||
| 1 | Anti-CD3 PCP | Anti-CD4 APC | Anti-CD45RA FITC | Anti-CD45RO PE |
| 2 | Anti-CD3 PCP | Anti-CD4 APC | Anti-CD45RA FITC | Anti-CCR7 PE |
| 3 | Anti-CD3 PCP | Anti-CD4 APC | Anti-CD27 FITC | Anti-CD28 PE |
| 4 | Anti-CD3 PCP | Anti-CD4 APC | Anti-CD25 FITC | Anti-CD127 PE |
| 5 | Anti-CD3 PCP | Anti-CD4 APC | Anti-CD45RA FITC | Anti-CD31 PE |
10 uL of antibody was used except for anti CD127 PE (2 uL). 100 uL of washed blood was added to each tube, the tubes were mixed and incubated at 4° C for 20 minutes in the dark. 2 mL of FacsLyse (Becton Dickinson (BD), Oxford, UK) was then added, the tubes were mixed and incubated for 10 minutes at room temperature in the dark. The cells were washed twice in PBS/Azide (600g for 90 seconds) and re-suspended in 1mL of Cellfix (BD, Oxford, UK). Samples were acquired on a FACS Canto (BD, Oxford, UK) within 24 hours and analysed using BD Diva software.
Th17/T regulatory cell assay
Th17 and T regulatory cell numbers were enumerated by the Becton Dickinson (Oxford, UK) Human Th17/Treg phenotyping kit according to manufaturer’s instructions. 20,000 – 30,000 events were acquired on a FACScalibur (Becton Dickinson, Oxford, UK) using Cell Quest Pro software.
NK cytotoxicity assay
NK cytotoxicity was evaluated as previously described [19]. PBMC’s were separated using Leucosep tubes (Oxford Immunotec, MA, USA), washed once in PBS and then incubated in plastic flasks for 1 hour at 37o C in 5% CO2. The cells were then washed and counted. The target cell was DIOC labelled K562 cells in log phase. The cells were mixed at ratios of 50:1, 25:1 and 12.5:1 (effector: target ratios) and incubated with 1/40 PI for 2 hours at 37C in 5% CO2 . After two hours, the cells were acquired on a FACS Canto using the Diva software. At least 10,000 events were counted.
Th1/Th2 assay
A whole blood method was utilised [20] for the enumeration of Th1/Th2 cell cytokines. Briefly, whole blood was diluted in culture medium, Golgi plug and Golgi stop (Becton Dickinson, Oxford, UK) were added and then cells were incubated with inomycin and polymyristate Acetate (PMA) overnight at 37C in 5% CO2 . The cells were then stained using the Fix and Perm protocol (Caltag Medsystem, Buckingham, UK), using CD3 and CD8 to identify the T cells and then staining for intracellular Interferon gamma (IFNγ), Tumour Necrosis Factor alpha (TNF-α), Interleukin 10 and Interleukin 4. 20,000 cells were acquired within 2 hours on a FacsCanto using Diva software.
RESULTS AND DISCUSSION
General results
All patients returned normal blood investigations for haematological, biochemical and thyroid dysfunction. Tests for organ specific autoimmunity and gluten sensitivity were negative, apart from two patients who had anti-Thyroid Peroxidase antibodies but normal thyroid function tests. The ESR and CRP were used to check for underlying inflammation and both were within the normal range in all the patients. Interestingly, two patients had mannose binding lectin deficiency, while one patient had a mild IgA deficiency (IgA = 0.74 g/L).
Cytokine measurements
Table (2) details the results of the serum cytokine analysis of patients with CFS and the healthy controls. For values below the measureable range we used 0 as the value in all statistical evaluations. These were particularly seen in the IL22 and IFN gamma results.
Table 2: Comparison of Cytokine levels in CFS patients and Healthy Controls using non-parametric Mann-Whitney Rank Sum test. P <0.05 is considered significant, marked by an asterix *. Units are in pg/ml except IL 22 and IL27 which are in ng/ml.
| Cytokine | Healthy Controls (n = 24) Mean(25-75 Percentiles) | CFS patients (n = 32) Mean(25-75 Percentiles) | P value |
| IFNγ | 5.19 (0.00 – 6.59) | 7.65 (0.34 -10.59) | 0.098 |
| IL 10 | 1.99 (0.00 – 1.77) | 1.899 (0.00- 2.65) | 0.331 |
| IL 12 p70 | 7.40 (2.39 – 9.47) | 17.92 (0.00 – 22.38) | 0.002* |
| IL 15 | 2.40 (0.00 – 2.79) | 3.07 (0.00 – 5.94) | 0.246 |
| IL 22 | 0.04 (0.00 – 0.058) | 0.096 (0.00 – 0.16) | 0.015* |
| IL 2 | 2.65 (0.00 – 4.45) | 3.31 (0.00 – 4.88) | 0.397 |
| IL 21 | 21.06 (9.51 – 23.16) | 33.57 (15.71 – 44.27) | 0.021* |
| IL 6 | 5.45 (0.00 – 5.73) | 10.55 (0.00 – 16.82) | 0.094 |
| IL 27 | 0.56 (0.32 – 0.67) | 0.833 (0.44 – 1.11) | 0.014* |
| TNF-α | 5.1 (1.44 – 6.82) | 8.43 (3.25 – 9.63) | 0.022* |
Using the non-parametric Mann Whitney Rank Sum test we observed significantly increased levels of interleukin-12, 21, 22, 27and TNF alpha in our patients with CFS compared to the healthy controls. The levels of the other measured cytokines were not significantly different between the two groups. However, there is considerable overlap between the levels of cytokine between the patient and healthy group, suggesting that the use of these cytokines as diagnostic or prognostic indicators with a defined cut-off is unlikely, even in those cytokines significantly different between the two groups. Bearing in mind the wide and varied severity of CFS in this sample population, this is not surprising.
T cell phenotyping
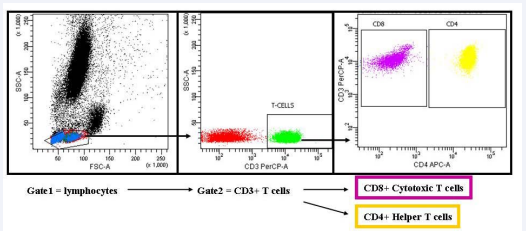
A sequential gating strategy was used to enumerate the various sub populations of T cells. Briefly, the FSC and SSC plot allowed definition of the lymphocytes, which were then divided into CD3 positive and CD3 negative cells (Figure 1).
Figure 1: T-cell gating strategy. Lymphocytes are selected in gate 1 using side versus forward scatter. The lymphocytes are further analysed for CD3 expression allowing T-cells to be selected in Gate 2. Gate 2 is further analysed for CD4 expression which allows helper T-cells to be selected (Gate CD4 yellow events). Any CD3+ cells not expressing CD4 are assumed to be cytotoxic CD8+ T-cells and are selected (Gate CD8 purple events). The CD4+ and CD8+ T-cells are further analysed, see Figure (2).
By gating on the CD3 positive cells the expression of CD4 was displayed and the gate put around either the CD4 positive population or the CD4 negative population (assumed to be predominantly CD8 positive cells, although some rare populations such as NK T cells and double negative T cells can’t be excluded). The specific markers were examined on these defined populations.
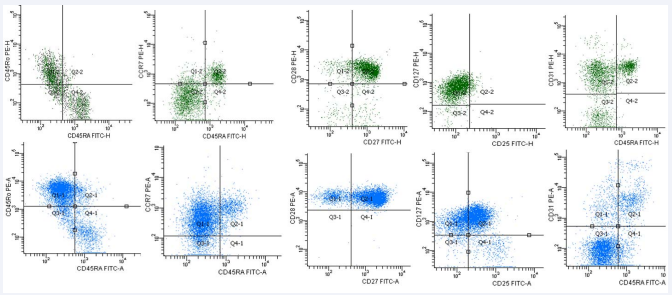
Significant differences between CFS patients and controls were detected in T-cell subsets defined by CD45RA and CCR7 expression (Figures 1 and 2 for gating strategy and Table 3).
Figure 2: Following separation of CD3+ T-cells into CD4- (assumed CD8+), shown in green, and CD4+ shown in blue, further analysis was performed. Expression of CD45RA CD45RO was carried out to identify memory and naïve cells. CCR7 CD45RA analysis allowed central memory, effector memory, naïve and effector cells to be separated. CD27 CD28 analysis allowed investigation of effector cells. Analysis of CD127 CD25 was used to identify T regulatory cells, activated T-cells, SLECs and MPECs. Recent thymic emigrants were analysed using CD45RA CD31. mem – memory. SLEC – short lived effector cells. MPEC – memory precursor effector cells. See text for full details.
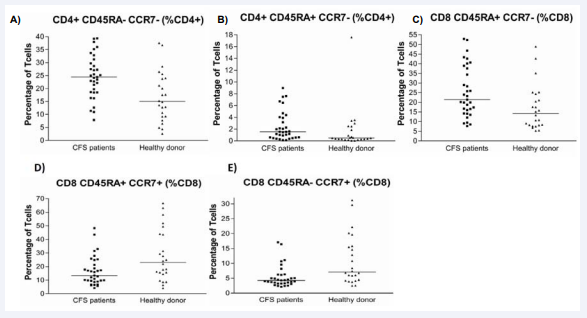
Figure 3: Graphs summarizing significantly different T-cell (CD3+) sub-populations as a percentage of either CD4+ or CD8+ T cells. A) TEM cells. B) T helper effector cells. C) Cytotoxic effector cells. D) Cytotoxic naïve cells. E) Cytotoxic TCM. For details see main text.
CCR7 is a chemokine receptor that mediates lymph node homing [21]. CD45, which is expressed by all lymphocytes has two isoforms, CD45RO expressed on memory T-cells and CD45RA on naïve T-cells [22]. The combination of these markers allows separation of naïve T-cells (CD45RA+CCR7+) from effector T-cells (CD45RA+CCR7-) and also effector memory T-cells - TEM (CD45RA-CCR7-) from central memory T-cells TCM (CD45RA-CCR7+). TEM are primed to go to sites of effector function, such as infection, and upon antigen encounter they express high levels of β1 and β2 integrins, which are necessary for migration to inflamed tissues. Upon antigen exposure, TCM migrate to lymph nodes where they are effective at activating dendritic cells. Upon subsequent encounter with antigen, TCM lose CCR7 expression and become effector memory cells and effector T-cells [23].
CFS patients had significantly greater numbers of effector cells, including: CD4+ TEM (CD4+CD45RA-CCR7-); T helper effector cells (CD4+CD45RA+CCR7-); and cytotoxic effector T-cells (CD8 CD45RA+CCR7-). Moreover, CFS patients had significantly reduced numbers of CD8+ lymph node homing T-cells, specifically the cytotoxic naïve T-cells (CD3+CD8 CD45RA+CCR7+) and cytotoxic TCM (CD3+CD8 CD45RA-CCR7+) (Table 3).
Table 3: Summary of the significantly different T-cells sub-populations. Parametrically distributed data was analysed using student T-test (first row). Non-parametrically distributed data was analysed using Mann-Whitney U tests, all other rows. CTL - Cytotoxic T-cells.
| Cell surface markers | Percentage of | CFS Patients (n=33) | Healthy Controls (n=24) | % diff | P-Value | ||||
| Mean | Min-Max | SD | Mean | Min-Max | SD | ||||
| CD3+CD4+CD45RA-CCR7- T helper Effector Memory cells | Lymphocytes | 8.1 | 0.09 – 13.5 | 2.9 | 5.6 | 0.8 – 11.6 | 3.0 | 44 | 0.002 |
| T-cells | 13.0 | 5.2 – 21.5 | 4.0 | 8.9 | 1.4 – 17.0 | 4.7 | 22 | <0.001 | |
| T helper cells | 24.6 | 7.9 – 39.3 | 8.3 | 16.8 | 2.6 – 37.6 | 9.7 | 46 | 0.002 | |
| Cell surface markers | Percentage of | CFS Patients (n=33) | Healthy Controls (n=24) | % diff | P-Value | ||||
| Median | 25 % quartile | 75% quartile | Median | 25 % quartile | 75% quartile | ||||
| CD3+CD4+CD45RA+CCR7- T helper effector cells | Lymphocytes | 0.4 | 0.2 | 1.2 | 0.2 | 0.09 | 0.4 | 100 | 0.014 |
| T-cells | 0.7 | 0.4 | 1.8 | 0.3 | 0.2 | 0.8 | 133 | 0.016 | |
| T helper cells | 1.5 | 0.6 | 3.9 | 0.5 | 0.3 | 2.2 | 200 | 0.015 | |
| CD3+CD8 CD45RA+CCR7- Cytotoxic effector T-cells | Lymphocytes | 6.1 | 3.9 | 10.1 | 3.8 | 2.1 | 6.1 | 60 | 0.032 |
| T-cells | 10.3 | 5.6 | 15.5 | 6.3 | 3.1 | 10.3 | 63 | 0.029 | |
| CTL | 21.4 | 15.5 | 37.9 | 14.3 | 8.2 | 22.8 | 50 | 0.008 | |
| CD3+CD8 CD45RA+CCR7+ Cytotoxic naïve T-cells | Lymphocytes | 3.0 | 2.1 | 7.3 | 5.7 | 3.6 | 11.2 | -47 | 0.021 |
| T-cells | 4.8 | 3.8 | 11.2 | 10.3 | 6.5 | 17.0 | -53 | 0.016 | |
| Cytotoxic T-cells | 13.4 | 9.4 | 22.1 | 23.0 | 15.0 | 43.8 | -42 | 0.031 | |
| CD3+CD8 CD45RA-CCR7+ Cytotoxic central memory T cells | Lymphocytes | 1.2 | 0.6 | 1.4 | 2.3 | 1.2 | 3.9 | -92 | 0.003 |
| T-cells | 1.9 | 1.6 | 2.4 | 3.4 | 1.9 | 6.5 | -44 | 0.003 | |
| CTL | 4.2 | 3.3 | 6.1 | 7.1 | 4.3 | 15.7 | -41 | 0.002 | |
Importantly, these differences were consistent, regardless of whether the subset was analysed as a proportion of all lymphocytes, a proportion of T-cells or a proportion of helper or cytotoxic T-cell.
Th17/T regulatory cells, Th1/Th2 and NK cytotoxicity
There were no significant differences in the numbers of Th17, T regulatory cells, Th1/Th2 intracellular cytokine populations between the patients with CFS and the healthy controls.
Many researchers including ourselves have summarized the various abnormalities of the immune system in patients with CFS [24,25]. Unfortunately the medical literature is replete with contradictory results. This may be related to several factors. Foremost amongst these would be the previous use of criteria that may not have stringently excluded fatigued patients with psychiatric comorbidity. In some cases fatigued patients without a significant delayed post exertion malaise, which is the most important symptom of CFS/ME, were also excluded. Furthermore, these reports investigated patients from differing countries and thus differing physical and microbial environments. It is therefore quite possible that the CFS/ME in these areas were caused by differing aetiological factors that may have contributed to the wide array of immune abnormalities. Other factors of importance would include the differing duration of disease suffered by patient as well as the use of different immune assays for the same analytes. Moreover, there is a strong possibility of non uniformity in the time of sample collection and the state of the patient’s mental and physical health at the time of sampling. Further problems relate to the degree of effort required for patients to attend for blood sampling and the quality of the patients previous night’s sleep. In the present study all patients were stable in regard to their CFS/ME, had no evidence of significant anxiety and depression and had blood taken in the afternoon having been rested for at least 30 minutes beforehand.
The most consistent abnormality reported in patients with CFS/ME relates to reduced NK activity [26]. Cytokine dys-regulation has also been documented but the findings are often contradictory [27-29]. Early studies had suggested increased pro-inflammatory cytokines [30] but others have found no difference [31] or increases in anti-inflammatory cytokines such as TGF beta [32]. Increases in the latter would favour T regulatory function but our current results do not show any changes in T regulatory cells or the other major T cell subsets. This would be in keeping with the clinical picture in patients with CFS/ME who do not have clinical evidence of systemic or organ specific autoimmunity or significant cellular infections. While the level of TNF alpha was raised in our patients, IL6 and IL1beta levels were normal. These results would be consistent with the normal levels of CRP in our patients.
Many patients with CFS experience flu- like symptoms such as myalgia. This has led some to suggest impaired cellular immunity in this condition. In this regard an alteration in the balance between Th1 and Th2 T cells has been suggested [20]. While other T cell changes have been documented, these have been inconsistent (reviewed in [24]). Nonetheless, alterations in T cell populations may result as a consequence of cytokine dys-regulation. These in turn may impact on B cell development and function.
In an attempt to address the problem of a heterogeneous patient population, our study investigated only patients with CFS fulfilling the Fukada, Oxford and Canadian and ICC diagnostic criteria. Additionally, we used our own stringent criteria for defining patients with CFS/ME and included only those having 10+ from 13 points [18]. In the cytokine analysis, methodological variables were addressed by the elimination of a stimulation step and the use of neat serum in the assays as we expected lower values than if the peripheral blood cells had been stimulated. Overall our present results suggest a subtle diffuse impairment of the immune system in CFS/ME. As such we have noted patients with CFS/ME to have significantly increased levels of IL12, Il21, IL27 and TNF alpha and with increased effector T cells. Clinically it is possible that this may leave patients with a subtle predisposition to recurrent viral infections or viral re-activation without evidence on routine clinical evaluation and using conventional tests of immune function.
We have recently reviewed the literature linking immune dysfunction in patients with CFS and an underlying persisting viral infection [25]. A range of viruses have been investigated in this regard and until recently both serological analyses as well as molecular tests enumerating viral copy numbers have failed to showconsistent differences in patients with CFS compared to healthy controls [33]. However Lerner et al. [34], have shown increased EBV viral copy numbers in patients with CFS although this was mainly as a latent infection with little evidence of BZLF1 expression. More definite evidence of EBV infection was reported more recently [35]where impaired EBV specific B and T memory cell responses were also noted. Interestingly, it has been shown EBV dUTPase are capable of stimulating inflammatory cytokine production and reducing T cell proliferation induced by mitogenic stimulation [34]. Thus actual viral replication is not critical for EBV induced immune dysfunction. In this light our results are interesting - showing that the cytokines involved in promoting antiviral immunity via the cellular immune system are increased in those with CFS; increased levels of IL12, IL21, IL22 and IL27. It will be interesting to see whether the levels of these cytokines are altered by variations in the clinical state of the CFS.
IL12 and IL27 are both members of the IL12 family of cytokines, which also include IL23 and IL35, and their main role is in the differentiation of T helper cells. They are structurally and functionally related and have both pro- and, in the case of IL27 anti- inflammatory effects (reviewed in [36]). IL12 is a disulphide linked heterodimeric 70kDa cytokine, formerly called cytotoxic maturation factor or natural killer cell stimulatory factor (NKSF). It is a pleiotropic cytokine mainly produced by monocytes, macrophages and dendritic cells in response to bacterial products or by contact with activated T cells. It causes interferon gamma production by B cells as well as inducing T cell and NK cell proliferation and cytotoxicity. The significantly increased levels of IL12 observed in the CFS patient population examined is interesting when related to previous reports of reduced NK cytotoxicity [24] and of a Th2 biased immune system in CFS patients. [20]. Regardless, we speculate whether the IL12 receptor in patients with CFS is functionally impaired in specific cell types. Alternatively, the B cell dys-regulation in those with CFS may allow the development of anti-cytokine antibodies that may impair the functioning of specific cytokines. Both suggestions would explain the normal levels of IFN gamma we observed as well as the absence of any alteration in NK cell cytotoxicity. Clinically, patients with CFS/ME do not manifest any of the consequences of impaired NK cell function in terms of increased frequency of significant viral infections or neoplastic growths. Thus our results are more in keeping with the clinical situation in CFS/ME.
In regard to B cells, IL12 has been shown to inhibit B cell differentiation to germinal centre cells and promote differentiation into short lived plasmablasts [7] . Impaired IL12 activity may thus explain our previous finding of reduced plasmablasts in those with CFS [8]. We are presently investigating anti-cytokine antibodies in patients with CFS.
IL21 has a key role in the development of high affinity B cell clones in the germinal centre (GC) via its expression by T follicular helper cells (TFH). IL21 promotes both immunoglobulin isotype switching, particularly to IgG4, and also immunoglobulin production generally by B cells. It is also involved in the maintenance and function of CD8(+) memory T cells and natural killer cells. One principal non-redundant role of IL-21 is the promotion of B-cell activation, differentiation or death during humoral immune responses. Recently it has been shown in a mouse model that IL21 appears to have a role in the production of regulatory B cells that have a key function in producing IL10 and regulating auto-reactive T cells [37].
The increased level of interleukin-21 in our patients raises the possibility of impaired B-cell maturation. In this regard our previous work has shown increased numbers of the more immature B cells[8]. It is possible that some B cells may escape rigorous negative selection. As such, we speculate whether patients with CFS have an increased burden of immature auto-antibody producing B cells. The clinical improvement in CFS patients after rituximab treatment [11] lends some weight to this suggestion. However, immunoglobulin levels overall in CFS patients are not generally reduced [8] which explains why these patients do not succumb to those infections seen in the recognized antibody deficiency diseases.
IL-27 is essential for the function of TFH cells and for germinal cell responses. This may be via IL-27 mediated T cell production of IL-21, which as mentioned previously is a known autocrine factor for the maintenance of follicular helper T cells (TFH cells), in a STAT3-dependent manner. IL-27 also enhances the survival of activated CD4+ T cells and the expression of TFH cell phenotypic markers. IL-27 has a non-redundant role in the development of T cell–dependent antibody responses [38] and thus its increase in CFS patients could impact on antibody responses.
It is not known whether the above cytokines fluctuate in level with disease severity or how stable they are within an individual patient over time. Longitudinal studies will be required to investigate this and to correlate levels with cellular aspects of the immune system. Another possibility is that there is a defect in some common aspect of the IL12 family of cytokines. We are therefore looking into investigating serum levels of IL23 and IL35.
In a recent study characterizing immune cells in patients with CFS, significant differences between different cell populations were detected [39]. pDCs and immature B cells were decreased in number in CFS patients compared to controls, while memory B cells and FoxP3 T regulatory cells were increased [39]. Interestingly, NK cell lysis of K562 tumour cells was decreased while degranulation and IFNγ production by the NK cells were increased, perhaps due to frustrated inability to lyse the K562 cells. The study also examined cytokines, finding no difference between patients and controls in IL-2, IL-4, IL-10, IL-7A, IL-6. However, in the CFS patients, IFNγ was significantly reduced and TNFα was significantly increased compared to controls. In our study we have found that IL-12, IL-21, IL-22, IL-27 and TNFα are significantly increased in CFS patients. The precise cause for these differences is unclear and along with the considerations detailed at the beginning of this discussion other factors would include serum levels of vitamin D which were low in many of our patients and were likely normal in the patients in Australia [39]. Vitamin D has a profound effect on several aspects of the immune system most notably on T cells [40], and needs consideration in future studies.
In the light of the above cytokine results it is perhaps not surprising that we found alterations in the T cell populations. All these cytokines play key roles in T cell development or function. However, the cause of the abnormalities is unclear. The CD8+ T-cell results suggest that CFS patients have more CD8+ effector-type T-cells (CCR7-) in the peripheral blood, and have reduced numbers of CD8+ T-cells with CCR7+, which are capable of homing to the lymph nodes in the periphery. There are two possible explanations. The first is that the CD8 CCR7+ T-cells have migrated to the lymph nodes, and so are no longer in the periphery, suggesting that some antigenic stimulus, such as infection, or auto-antigen is activating the T-cells. In this case the CD8 CCR7+ T-cells are likely to have encountered secondary antigen and be in the process of losing CCR7 expression, developing into cytotoxic effector T-cells (CD8 CD45RA+CCR7-), explaining the increase of these cells in CFS patients. Alternatively, it is possible that there is impaired CCR7 expression that reduces lymph node homing which in turn leads to reduced control of viral infections at these sites. These findings are in keeping with a recent study, which published reduced CD3+ CD8+ CD45RA-CCR7- CD27- CD28+ effector memory cells, 4.3 % in CFS patients compared to 6.7 % in healthy controls, p = 0.0046 [12].
The situation regarding the CD4 T cells and CCR7 expression is similar to the CD8 cells. We speculate whether patients with CFS have a global impairment of lymph node homing receptors that prevents adequate containment of lymphotrophic viruses by CD8 T cells and at the same time is associated with altered B cell function by CD4 T cells. Intriguingly, this pattern of increased TEM cells and reduced TCM cells has been seen in the peripheral blood and cerebrospinal fluid of patients with inflammatory neurological disease [41]. This is particularly interesting, as white matter lesions are increased in those with CFS and the post-exertion malaise that is seen frequently in those with CFS is also seen in patients with multiple sclerosis. It is tempting to postulate that the T cell alterations allow autoimmunity to develop against neural auto-antigens, resulting in subtle brain inflammation and contraction of brain volume [42]. However, our early un-published work has not so far shown evidence of a significant autoimmunity to whole neurones on direct immune fluorescence or on western blotting.
Recent reports have shown several changes to immune cells in patients with CFS/ME. In this regard Hardcastle et al. [43], have recorded differences in gamma delta 2 T cells, iNKT cells, effector memory T cells as well as naïve CD8+ T cells in patient with CFS/ME compared to healthy controls. Later results from this group showed reduced expression of CD27 on CD8 T cells [44]. Our results are different from these and we did not observe a significant decrease in NK cells or raised T regulatory cells in CFS patient compared to healthy controls compared to other groups [12]. The several factors detailed previously are likely important in producing the different results seen in the different studies. Particularly important would be the differences in laboratory methodology and certainly in relation to measuring the different types of T cells. Regardless, it is quite possible that it is precise causative factors of CFS/ME that influence the pattern of T and NK cells and their memory and activation status and thus it is not surprising that results are different in each of the different research centers.
CONCLUSION
In conclusion, patients with CFS have impaired production of those cytokines that regulate cellular immunity and lymph node function. This is associated with, and may be caused by, altered T cell lymph node homing. Whether these alterations in the immune system leave patients with CFS susceptible to viral infections or a reduced ability to clear viral infections effectively is open to debate. It is also unclear whether these abnormalities are the cause of CFS/ME symptoms or are the consequence of the physical, mental and emotional stress caused by the condition and the associated sleep problems. Regardless, it is tempting to speculate whether the subtle abnormalities of the immune system in CFS/ME may be due to one or more viruses contributing to low grade inflammation that may promote oxidative damage to self proteins leading in turn to an increased tendency to autoimmunity [45,46]. Clearly much more work needs to be done to investigate this possibility and to ascertain the precise link between abnormalities of the immune system with viral infection(s), endocrine dysfunction and stress and sleep disturbance.
ACKNOWLEDGEMENTS
The authors would like to thank Sarah Yates for her technical expertise when measuring the sera cytokine levels. This work was made possible by funding from the ME solutions charity and the state of Abraham Goudsmit.
Ethical and research approval
This research project was granted ethical approval by the Central London REC 1 Research Ethics Committee (Rec No: 09/ H0718/54) and approved by the research and development department at St. Helier Hospital, R&D No: 015/2009/DPH.
Contribution of authors
Brian Ford and Alison Bradley did all the cellular laboratory work. Alison Bradley, Amolak Bansal and Brian Ford all contributed equally to the production of the manuscript.