Migraine is a Multifaceted Condition That is More Than Just a Headache: A Review
- 1. Institute of Human Genetics, University of Jammu, India
- 2. Department of Human Genetics, Cluster University Srinagar, India
- 3. Department of Zoology, University of Jammu, India
- 4. Department of Zoology, Lovely Professional University, India
- 5. Department of Psychology, University of Jammu, India
- 6. Department of Lifelong Learning, University of Jammu, India
- 7. Department of Neurology, Super Specialty Hospital, India
Abstract
Background: Migraine is a complex and common neuro-vascular disorder with a distinctive periodicity and is described by a reduced level of neuronal hyper-excitability. It is imperative to comprehend the complexity of the disorder from the standpoint of pathological mechanisms, genetic variable associations that contribute to disease severity, and a debatable phenotype i.e., inflammation which might contribute to the chronification of the condition, migraine as a channelopathy, and constellations network of co-morbidities.
Aim: In the present review, we aim to better understand the complexity of migraine by consolidating the provided data.
Material & method: Online repositories including PubMed, Web of Science, and Google Scholar were used for the literature survey and relevant articles were utilized to comprehend the complexity of migraines for better understanding.
Result: Migraine is a complex neurological disorder featuring a heap of candidate gene alterations that predispose by altering the function of channels in the brain, hindering the process of inflammatory homeostasis, microglial activation, and complex interaction with different diseases.
Conclusion: In conclusion, featured with a wide abnormal pattern of phenotype enlightened the complicated nature of migraine, and it can’t be said that migraine is not just a headache but more than it.
Keywords
• Migraine
• Channelopathy
• Neurogenic Neuroinflammation
• Neuronal hyper-excitability
CITATION
Sudershan A, Bhagat M, Bharti S, Bhagat S, Sachdeva P, et al. (2023) Migraine is a Multifaceted Condition That is More Than Just a Headache: A Review. J Chronic Dis Manag 7(2): 1033.
INTRODUCTION
Chronic pain has a negative impact on one’s quality of life but it is undeniable that pain perception (acute pain) is important as it is the “survival mechanism”. Therefore, defined as an adaptive response that helps to protect the body from injury and stimulates the repair system of the injured tissues [1]. But the pain (chronic pain) at any time is still a serious medical challenge, affecting approximately 30% of the population [2,3]. One such example is migraine, which is defined as a complex, polygenic/ multifactorial, dysautonomic, common, and chronic neurovascular disorder featuring unilateral head pain [4]. It is responsible for high disability-adjusted life years (DALYs), an overall high prevalence (15.75%) (Healthdata.org) with varied prevalence rates between sexes i.e., the female occurrence is more (12-17%) than male prevalence [5,6]. Migraine patients have significantly higher visits to healthcare providers, and diagnose the condition according to the ICHD-3 are unilateral headache, vomiting, phono-phobia, photophobia, and some patient also experiences stomach and abdominal pain, dizziness, pale skin color, and tiredness. For decades, various physiological and pathological pathways have been established, including Cortical Spreading Depression (CSD), the trigeminovascular system, inflammation, and brain structural alteration[13-16], but the precise underlying processes responsible for the different characteristics of the condition, such as reduced neural threshold and chronification, remains elusive.
Finding the cause of this complex condition is a tremendous challenge as it’s the result of various influences, such as genetic defects, epigenetics, environmental factors, and their interactions. The motivation for writing this review primarily stems from its complexity, and integrating the various risk attributes that have been identified over the last few decades into a single draft will probably benefit in a better understanding of this complex disorder. Also, the fact that cannot be neglected is that migraine is a significant cause of disability but many continue to neglect it and seek medical attention only when they have a severe attack or a chronic progression. This lack of understanding among individuals is also a matter of concern.
Therefore, in this culminating convection, we tried to grasp the various aspects of this neurological condition and tried to provide the information to the reader who says that “migraine is just a headache” and not to be worried about.
MATERIAL AND METHOD
A structured survey of research articles and review of the literature was done in the electronic databases of Google Scholar, PubMed, Springer, and Elsevier of the last two decades using the keywords “migraine as a neurological disorder, migraine, and inflammation, role of immune cells in migraine, pro-inflammatory molecules activation, migraine, and neuro-inflammation, a risk factor associated with migraine, migraine as a disease of the channel, migraine, and channelopathy, channels abruption in migraine, epigenetic, and migraine, epigenomic association and migraine, genetic polymorphism and migraine, genetic architecture, and migraine. Only articles published in the English language were evaluated using the linguistic filters as a factor for publication selection primary and secondary research sources included scientific work, meta-analysis, systematic reviews, and peer reviews. The study excluded data that was unpublished, incomplete, or only partially available, as well as publications in several languages. Because partial and missing data are not included in the study, there is no such detrimental effect and we did our utmost to eliminate any undesirable characteristics. This narration includes a significant quantity of current material. The relevant writers independently verified the data’s validity.
Background
Because of migraine’s complicated nature, researchers still try to figure out the various aspects of the disease, including lower neuronal threshold, chronic progression, genetic susceptibility/ genetic predisposition, and phenotypic variability. For decades, various physiological and pathological pathways, such as cortical spreading depression, the trigeminovascular system, inflammation, and brain structural changes, have been established, but the fundamental underlying cause of the complexity has remained unknown. Discussing the various aspects of the disease here in this piece of the review may aid in gaining a thorough understanding of the complexity of the disorder.
Genetics of migraine
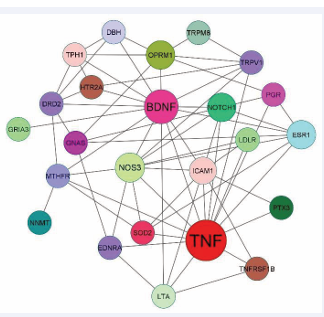
It is well known and recognized that a phenotype is encoded by the genes and alteration in the same leads to the abrupt phenotype i.e., disorder and migraine is an astonishing example of polygenic inheritance i.e., caused due to a plethora of changes in many genes (www.genecards.org/).In human disease genes, there is a wide range of allelic variations, and one such prevalent type of variation is single nucleotide polymorphism (SNP), a fascinating genomic “marker” that acts as a causative variation by altering the susceptibility [17]. Numerous candidate gene association studies (CGAS) have been performed for migraine which aimed to find risk variations associated with the disease [147,148,150]. This inexpensive, quick-to-conduct approach i.e., a candidate gene association study has provided numerous candidate variants [Table 1]. Using the String-Cytoscape tool (String-Database.Org) interaction between the different candidate genes has revealed that the BDNF and TNF-α protein shows the highest interaction with the highest node degree i.e., 14 undirected edges. (Figure 1).
Figure 1: Candidate gene-gene interaction: Using string database, the interaction between candidate gene product was created. This complex protein protein interaction revels that the BDNF and TNF-α shows the highest node degree of about 14 undirected edges. This intricate network revealed the disease’s complexity as well as the strong genetic component in determining susceptibility.
Table 1: Candidate genes significantly associated with migraine from different demography.
|
Gene |
Function |
rs’ ID/Loci |
Study |
Case |
Control |
Pop./Ethnic |
|
ETA |
Vasoconstrictor |
-231 A/G |
Cohort |
1048 |
140 |
France |
|
5-HT2A |
Serotonin transporter |
T102C |
CC |
61 |
44 |
Turkey |
|
LDLR |
Receptor-mediated endocytosis |
G142A |
CC |
140 |
200 |
Italy |
|
eNOS |
Vascular smooth muscle relaxation |
Glu298Asp |
CC |
56 |
125 |
Italy |
|
TPH |
Catalyzes the biosynthesis of serotonin |
A218C |
CC |
55 |
62 |
Turkey |
|
GNAS1 |
inhibit the adenylyl cyclase-stimulating activity of guanine nucleotide-binding protein G(s) |
T393C |
CC |
365 |
347 |
Spain |
|
MTHFR |
Folate biosynthetic pathway |
677C>T |
MA |
6446 |
24578 |
n. CP |
|
Notch |
Development of cell |
rs1043994 |
CC |
258 |
247 |
Australian |
|
HRH3 |
Histamine signaling |
A280V |
CC |
147 |
186 |
Mexico |
|
OPRM1 |
Receptor for endogenous opioids |
A118G |
Cohort |
153 |
- |
Australia |
|
TNFRSF1B |
TNF-α Receptor |
rs5745946 |
CC |
416 |
415 |
China |
|
PRNP |
Neuronal development and synaptic plasticity |
V129M |
CC |
384 |
185 |
Italy |
|
GRIA3 |
Glu. Receptor excitatory synaptic transmission |
rs3761555 |
CC |
472 |
472 |
CP |
|
ICAM1 |
Cell proliferation, differentiation, motility |
rs5498 |
CC |
114 |
125 |
AP |
|
TNF-β |
Inflammatoryand antiviral responses, |
252 A>G |
MA |
5557 |
20543 |
Asian |
|
uPAR |
Receptor for urokinase plasminogen activator |
rs344781 |
CC |
103 |
100 |
Iran |
|
ESR1 |
ER- ligand-activated transcription factor |
594G>A |
MA |
2293 |
2026 |
CP |
|
ESR1 |
ER- ligand-activated transcription factor |
325C>G |
MA |
2027 |
1919 |
CP |
|
ESR1 |
ER- ligand-activated transcription factor |
XbaI |
CC |
102 |
115 |
Indian |
|
PGR |
TF activity & signaling receptor binding. |
rs1042838 |
CC |
380 |
185 |
Italy |
|
SOD2 |
Protein binds to the superoxide byproducts |
rs4880 |
Cohort |
490 |
246 |
Italy |
|
PTX3 |
Fibrocyte differentiation & Inflammation |
rs3816527 |
CC |
103 |
148 |
Iran |
|
TNF-α |
Proinflammatory reaction |
rs1800629 |
MA |
|
|
nCP |
|
NNMT |
Regulating cellular methylation potential |
rs694539 |
CC |
433 |
229 |
Turkey |
|
DBH |
Expressed in neurosecretory vesicles |
+1603C>T |
CC |
200 |
267 |
Turkish |
|
BDNF |
Catalyzes the conversion of dopamine to NE |
rs6265 |
MA |
1598 |
1585 |
CP |
|
BDNF |
Differentiation of neuronal cells and growth |
rs2049046 |
MA |
1598 |
1585 |
CP |
|
GABA |
Inhibitory neurotransmitter IN CNS |
rs1186902 |
CC |
197 |
298 |
CP |
|
eNOS |
Vascular smooth muscle relaxation |
-786T>C |
MA |
|
|
CP |
|
GABA |
Inhibitory neurotransmitter IN CNS |
rs3810651 |
CC |
197 |
394 |
Spain |
|
DRD2 |
A GPCR that inhibits adenylyl cyclase activity |
rs1800497 |
CC |
250 |
250 |
China |
|
TRPM8 |
Receptor-activated non-selective cation channel |
rs10166942 |
Cohort |
1904 |
|
Taiwan |
|
5-HTR6 |
Regulate cholinergic neuronal transmission |
rs770963777 |
CC |
92 |
100 |
AP |
|
TRPV1 |
Convey inf. about noxious stimuli to CNS |
rs8065080 |
CC |
46 |
50 |
Russian |
On the other side of the basic candidate gene association approach, a statistical high throughput association study i.e., Genome-Wide Association Studies (GWAS) is now the most frequent method for discovering highly significant common variations, and in migraine, various GWAS have been conducted from last few years[18,19]. Conducting GWAS for migraine, enlightened various aspects including the polygenic risk score (PRS), heritability, genetic predisposition, phenotypic variability, etc. Hautakangas and colleagues conducted a large meta-GWAS with a sample size of 102,084 cases and 771,257 controls, identifying 123 migraine risk loci, 86 of which are novel compared to the previous migraine meta-analysis, which yielded 38 loci [19].
The findings of the GWAS significantly improved migraine genetic data and revealed that migraine susceptibility is conferred by a combination of many genetic variations with small effect sizes, as well as environmental factors. However, it is unavoidable that highly significant GWAS-SNPs with low penetrance do not aid in the identification or classification of a common and complex disorder; rather, they provide information about a specific observable intermediate trait [20]. Another astonishing feature related to genetics is the PRS, which has been enlightened by the large study including 1,589 families from Finland and the calculation of PRS for all 8,319 family members and 14, 470 individuals from the FINRISK population cohort. They found a significantly higher common variant burden in familial cases and also found a significant polygenic burden for different features of migraine [21].
Now the second issue is “What is the heritability of the condition?” which provides a glimpse of the relative significance of genes and environment to the variance of characteristics within and across populations [22]. Heritability appears to be due to a large number of weak associations with many genetic variants, rather than a few common risk variants [23]. Noble Topham and colleagues provided the evidence that the “MA population” is heterogeneous i.e., arises due to the interaction of genetic and environmental factors with a crude recurrence risk to siblings of probands were 2.7-fold higher in three-generation compared with two-generation MA families and 4.8-fold higher compared with one generation MA families [24]. These results showed the numerous features of migraine-related hypotheses such as the first-degree relatives of individuals with MA show a considerably higher risk.
Enclosing the section, migraine susceptibility is influenced by several common variations, implying that genetics plays a substantial role in defining susceptibility and making a person vulnerable to migraine. But still, there is a diverse important issue that is still unanswered such as “how likely is it that a person who has been diagnosed with the condition would have had possible variations found in the last 20 years”? Another question that remains unresolved after the larger meta-analysis is “how much genetic variation exists between MWA and MA?” [18,19]. Aside from sequence variations, there is also evidence that epigenetic factors may play a major role in determining migraine susceptibility and development thus increasing the complexity of the risk of the disorder [25].
Epigenetic and migraine
The introduction of a new notion of “heritable change in the genome that cannot be explained by mutation” has significantly changed the way things are about the subject [26] and is now referred to as “epigenetic”. The word “epigenetic” has expanded over time [27], and has made a significant achievement over the last two decades in understanding different genetic disorders. Epigenetics plays a pivotal role in a wide range of diseases including neurodegenerative disorder [28], and has advanced at a breakneck rate. Epigenetics in migraine is an emerging field and various research groups have started exploring epigenetics in migraine [29].
Histone modification which is the addition of acetyl groups from acetyl-CoA to lysine residues of histone-by-histone acetyl-transferase (HATs) [30] and removal of acetyl group by Histone acetyltransferase (HDACs) [31] is another exciting phenomenon which found to be signification associated with migraine. Different research groups [32-36] found that abrupt DNA methylation of the different genes and found significantly associated with migraine.
Another element of gene regulation is a small (~22 nucleotide) non-coding RNA called miRNAs that are associated with migraine [37]. Shreds of evidence from the serum miRNAs level studies, where it was found the level of miRNAs level in the serum of migraineurs during attacks and pain-free periods have altered values. Notably, the miR-34a-5p shows a 9-fold increase in expression [38], along with the elevated level of miR-155, miR-126, and Let-7g [39], hsa-miR-34a-5p and hsa-miR-375 in aura untreated patients as compared to control participants and aura treated patients [40]. Whereas miR-29c-5p and miR-382-5p showed modest 4.2 or 4.1 increases, respectively [38]. In order to establish a connection between inflammation and epigenetics, it has been evidenced that the proinflammatory cytokine TNF-α (Tumor Necrosis Factor-alpha) activates the miR-342, which is well known for its crucial role as an activator of NF-kB/p65 factor by destroying BAG-1, a negative regulator of NF-kB [41]. Recent meta-analyses of TNF-α have also shown a significant increase in the risk of migraine [42].
Discovering the association between miRNAs and migraine opens the doors for a new treatment strategy and this could be a game-changer in migraine miRNA pharmaco-epigenomics.
The miRNA pharmaco-epigenomics may provide new insights into individual drug heterogeneity and response that might lead to more effective treatments. Recently, much attention was paid to the therapeutic potential of miRNAs (miRNA medicinal products) in cancer [43], and neurodegenerative disorders [44]. Information has however just begun and new studies are needed for knowledge in the field of the therapeutic role of miRNAs, the drug ability of miRNAs; and the modulation effects of current abortive and preventative drugs on miRNAs in migraine [45].
Migraine as an inflammatory disease
Inflammation is a defensive mechanism with molecular and cellular activities responsible for limiting immediate harm to the system. This ensures the restoration of tissue homeostasis and the resolution of acute infection [46]. Uncontrolled inflammation on the other hand can develop chronic reactions leading to a range of chronic inflammatory disorders [47]. Neurogenic neuroinflammation migraine is characterized by inflammatory reactions in the trigeminovascular system’s central and peripheral components in response to abnormal neuronal hyperactivity or hyperexcitability [48]. Microglia cells (the immune member of the CNS immune system family) continuously check for infection and physiological changes in their environment thus helping in the maintenance of CNS homeostasis [49]. Activation of microglial has been investigated in migraine for decades [50,52]. Excessive and persistent microglial activation may be the trigger for the progression and transition of the migraine. The key ligand required for the activation of the microglial cell is CGRP (calcitonin gene-related peptide), which is released by the CSD from the trigeminal neurons. The increased CGRP levels rise during a migraine attack, most likely responsible for the release of inflammatory cytokines importantly TNF-α which is released in or near trigeminal ganglion neuronal cell bodies and may act as a neuronal signal enhancer, resulting in sensitization [48].
Activation of microglial is an important variable for the migraine progression, transition, and linking of the various candidate signaling pathways molecules which are reviewed in detail [51]. The likelihood that migraine will be classified as a neurogenic neuroinflammation condition is increased by the fact that these molecules are essential for the activation of microglial cells. This is further supported by the GWAS study, which identified several inflammatory genes, including TSPAN2, MEF2D, NLRP1, JAM3, and NOTCH4 [21].
Migraine as a channelopathies
Migraine is described as an episodic condition of abnormal brain excitability [53] and despite recent progress in migraine research, the pathogenic mechanism of migraine is related to the lower neuronal hyperexcitability, which remains a controversial subject among scientists. It is still under-recognized and inadequately treated and this is only due to the complexity that is involved in migraine pathogenesis, chronification, etc. It is well understood that ion channels are essential for the generation, representation, and modulation of the brain’s response to external stimuli and that when these channels work or behave abnormally, the risk of an abrupt phenotype increases. One such example is “abnormal brain excitability” which has been widely linked to the abnormal behavior of brain channels. The malfunctioning of channels leads to chronic neurological conditions and is broadly categorized under channelopathies.
Therefore, channelopathies are defined as the reason for the diverse category of neurological conditions caused by ion channel malfunction in biological membranes and organelles. Defects in many ion channels or transporters can frequently underlay a single neurological presentation. Various neurological conditions are already categorized under the class channelopathy [54-57], and there has also been an attempt to categorize migraine under channelopathy. Over the past two decades, various channels and their mutations have been recognized by different research groups and linked with migraine pathology [Table 2]. This channel dysregulation leads to episodic disturbances of the excitation inhibition balance and cortical network hyperactivity in response to particular migraine triggers serving as the ground for CSD ignition susceptibility [53]. Decreased neuronal hyperexcitability is a major consequence of abnormal behavior and cross-talk of mutated channels and opens the gate and provides a solid base for migraine is not just a headache but a complex channelopathy [58]. Several disorders are associated with migraine, and the mechanism that connects them is the abrupt channels [59-62]. Enclosing the paragraph, ion channel mutations can damage the entire nervous system if they are inherited [62] and these channelopathies are one of the diverse categories of neurological conditions and migraine i.e., “lower neuronal hyperexcitability disorder” fall under channelopathies.
Table 2: List of Genes that are activate during migraine in microglial cells.
|
Gene |
Protein |
Ligand/ Activator |
Function |
Brain Expression |
Activators |
|
CGRPR |
Calcitonin receptor like receptor |
CGRP |
The calcitonin-gene-related peptide (CGRP) receptor works with RAMP1 and the adrenomedullin receptor works with RAMP3 (By similarity). Together with RAMP2, this receptor binds to adrenomedullin CGRP. This receptor's action is mediated by G proteins that activate adenylyl cyclase. |
Hypothalamus, Spinal Cord & White Matter |
P38 phosphorylation & NF-kβ activation |
|
TNFR1 |
Tumor necrosis factor Alpha |
TNF-α |
It can induce cell death of certain tumor cell lines. It has the ability to increase cell proliferation and differentiation under certain conditions |
Hypothalamus, White Matter & Medulla Oblongata |
NF-kβ activation |
|
mGluR |
Glutamate metabotropic receptor |
Glutamate |
Ligand binding produces a conformational shift in G proteins, which stimulates signaling and affects the activity of downstream effectors such adenylate cyclase |
Pons, Medulla Oblongata & White Matter |
Hyperexcitability (enhance CSD) |
|
P2Y12Rs |
Purinergic receptor P2Y12 |
ATP |
Receptor for ADP and ATP coupled to G-proteins that inhibit the adenylyl cyclase second messenger system |
White Matter, Spinal Cord &Pons |
P38 phosphorylation |
|
NOTCH1 |
Notch receptor 1 |
Jagged-1 |
To regulate cell fate determination, it acts as a receptor for membrane-bound ligands Jagged-1 (JAG1), Jagged-2 (JAG2), and Delta-1 (DLL1). It forms a transcriptional activator complex with RBPJ/RBPSUH and activates genes of the enhancer of split locus after ligand activation through the released notch intracellular domain (NICD). |
Medulla Oblongata, Pons, & Basal Ganglia |
NF-kB translocation and enhance signaling |
|
P2X4Rs |
Purinergic receptor P2X 4 |
ATP |
This gene's product belongs to the ATP purinoceptor family. This receptor works as a calcium-permeable ligand- gated ion channel |
Cerebellum, White Matter & hypothalamus |
P38 phosphorylation & BDNF secretion |
|
CNR2 |
Cannabinoid receptor 2 |
2-AG |
Adenylate cyclase inhibition is mediated via a heterotrimeric G protein-coupled receptor for the endocannabinoid 2-arachidonoylglycerol. It's possible that it's involved in the inflammatory response, nociceptive transmission, and bone homeostasis |
Cerebellum |
MMPs (Matrix metalloproteinases) |
Table 3: Different channels that behaves abnormally and are responsible for the condition.
|
Gene |
Chr. Loc |
Protein |
Expression in brain |
Function |
|
CACNA1A |
19p13.13 |
Voltage-dependent P/Q-type calcium channel subunit alpha- 1A |
Cerebellum, Pons & White Matter |
VSCCs mediate calcium ion entrance into excitable cells and are also engaged in a number of calcium-dependent activities such as muscle contraction, hormone or neurotransmitter release, gene expression, cell motility, cell division, and cell death. |
|
ATP1A2 |
1q23.2 |
Sodium/potassium-transporting ATPase subunit alpha-2 |
Thalamus, Pons & Medulla Oblongata |
This is the active enzyme's catalytic component, which catalysis the hydrolysis of ATP as well as the exchange of sodium and potassium ions across the plasma membrane. This activity generates an electrochemical gradient of sodium and potassium, which provides energy for active nutrient transport. |
|
SCN1A |
2q24.3 |
Sodium channel protein type 1 subunit alpha |
Cerebral Cortex, Pons & White Matter |
Excitable membranes' voltage-dependent sodium ion permeability is mediated by Sodium channel protein type 1 subunit alpha |
|
TRPA1 |
8q21.11 |
Transient receptor potential cation channel subfamily A member 1 |
Hippocampal Formation & Cerebral Cortex |
Receptor-activated non-selective cation channel involved in pain detection and possibly also in cold perception, oxygen concentration perception |
|
TRPM8 |
2q37.1 |
Transient receptor potential cation channel subfamily M member 8 |
Mid brain |
It allows the entry of Na+ and Ca2+ ions to the cell, Depolarization |
|
KCNMA1 / BKCa |
10q22.3 |
Calcium-activated potassium channel subunit alpha-1 |
Cerebral Cortex, Basal Ganglia& Amygdala |
Potassium channel that is triggered by both membrane depolarization and a rise in cytosolic Ca++ and mediates K (+) export. The concentration of cytosolic Mg++ also activates it. Its activation diminishes the excitatory processes that raise cytosolic Ca++ levels and/or depolarize the cell membrane. |
|
KCNJ8 |
12p12.1 |
ATP-sensitive inward rectifier potassium channel 8 |
Hypothalamus, Thalamus & Cerebral Cortex |
Allow potassium to flow into a cell rather than out of a cell |
|
KCNK18 |
10q25.3 |
Potassium channel subfamily K member 18 |
Hypothalamus |
It is possible that this channel acts as a background potassium channel, determining the resting membrane potential and Calcium signals directly activate channel activity. |
|
PRRT2 |
16p11.2 |
Proline-rich transmembrane protein 2 |
Cerebellum, Cerebral Cortex &Hippocampal Formation |
It is engaged in synaptic transmission in the central nervous system, particularly hippocampal neurons, in presynaptic terminals, and plays a critical role in the last steps of neurotransmitter release, potentially through regulating Ca(2+)-sensing. In the cerebellum, may prevent the development of SNARE complexes and reduce short-term facilitation. |
|
Panx1 |
11q21 |
Pannexin-1 |
Hypothalamus, Basal Ganglia & Cerebral Cortex |
It is a structural component of gap junctions and hemichannels that are involved in ATP release and nucleotide permeation. It may also act as a Ca(2+)-leak channel to regulate ER Ca(2+) homeostasis. |
|
NMDA R/ GRIN2A |
16p13.2 |
Glutamate receptor ionotropic, NMDA 1 /Glutamate receptor ionotropic, NMDA 2A |
Cerebral Cortex, Hippocampal Formation & Hypothalamus |
NMDA receptor complex component that functions as a heterotetrametric, ligand-gated ion channel with high calcium permeability and voltage-dependent magnesium sensitivity. |
|
AMPA R/ GRIA1 |
5q33.2 |
Glutamate receptor 2 |
Cerebral Cortex, Hippocampal Formation & Cerebellum |
The glutamate ionotropic receptor is a type of glutamate receptor that is found in the brain Many synapses in the central nervous system use L-glutamate as an excitatory neurotransmitter. The excitatory neurotransmitter L-glutamate binds to the receptor, causing a conformational shift that opens the cation channel, converting the chemical signal to an electrical impulse. |
|
SLC6A4 |
17q11.2 |
Sodium-dependent serotonin transporter |
Midbrain ,Pons& Thalamus |
The major function of the serotonin transporter in the central nervous system is to regulate serotonergic signaling by transporting serotonin molecules from the synaptic cleft to the pre-synaptic terminal for re-use. Plays an important role in regulating the availability of serotonin to other serotonergic receptors. |
Migraine Co-morbidity
Another major issue to consider is co-morbidity, which is defined as the presence of two or more chronic conditions at the same time [63]. More than one-third of patients with migraines observed by primary care doctors found four or more chronic health conditions associated with migraine (Co morbidity) (Morbinet). There are several causes for such disease comorbidity with migraine, some of which include having similar pathophysiological mechanisms [64], common risk factors including common genetic variants, and common environmental risk factors.
The most widely used and validated recipe notably, a “literature survey” was first used to search for “migraine’s comorbidity”, which revealed that migraine is comorbid with various common and detrimental conditions which include temporomandibular disorders (TMD) [65], mood and anxiety disorders [66,67], restless legs syndrome (RLS) [68], alexithymia and post-traumatic stress disorder [69], depression [70], ischemic strokes [71], hypertension [72], asthma [73], dementia [74,75], cardiovascular disorders [76], sleep disorders [77], PFO (Patent Foramen Ovule) [78], gastroesophageal reflux, tooth wear, systemic inflammation of Behçet’s disease (BD) [79], irritable bowel syndrome [153], Chronic Fatigue Syndrome (CFS) where that mechanisms of migraine pathogenesis such as central sensitization may contribute to CFS pathophysiology [80]. It has been also shown that sleep fragmentation and oxygen desaturation may worsen migraine but migraine may help in reducing apneas by a more superficial sleep [152]. Patients with Sydenham’s chorea (SC), rheumatic fever (RF), and other basal ganglia disorders like essential tremor and Tourette’s syndrome experience migraines more frequently [81]. Furthermore, compared to controls, migraine patients had higher diastolic blood pressure, lower systolic blood pressure, and lower pulse pressure, according to the population-based study [82]. Ito and colleagues suggest that migraine-like PIH epileptic seizure (Post Ictal Headache: PIH) is related to particular regions of epileptogenic focus and that susceptibility to migraine headache predisposes to migraine-like PIH [83]. Additionally, studies have found that individuals who have a history of migraines without aura may also have probable analgesic-use headache (PAH) or probable analgesic-abuse headache (PAAH) which is defined as the transformation of episodic to chronic headache due to medication overuse, and probable chronic migraine (PCM) (>8 migraine attack for at least 3 months) [84].
In addition to the literature review, comorbidity data of migraine were retrieved from the large Spanish data repository which collected the data from the Information System for Research Development in Primary Care (SIDIAP), with a sample size of about 5.5 million people, or 74% of Catalonia’s population which was studied retrospectively in a population-based study (Morbinet.org). In this electronic record, diseases were coded using the International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD-10) system. With a sample size of around 167905 (4.1%), this Spanish registry represents migraine (ICPC2 code: N101) and formed an interesting multimorbidity network based on the disease’s co-occurrence rate, which is higher than expected based on its prevalence.
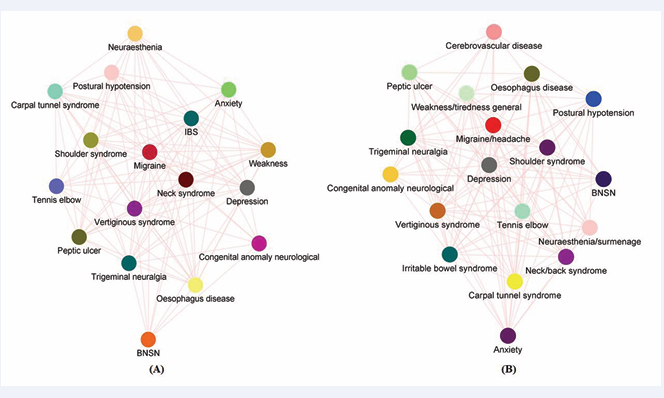
The Multimorbidity network has enlightened here on the numerous aspects of migraine features, including the fact that women have a more complicated multimorbidity network than men (Figure 2B)
Figure 2: (Morbinet): Network graph shows a complex interaction of different diseases with the migraine disorder. (A): Multimorbidity network adjusted for both sex female and all age group which shows 17 interconnected nodes with 112 edges and an average shortest path of 1.176. (B): Multimorbidity network adjusted for female sex found 18 nodes with 118 edges with an average shortest distance of 1.229.
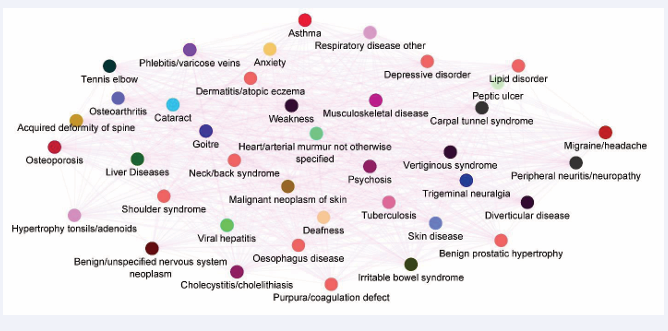
and that as age progresses, the multimorbidity network of migraine becomes more complex (Figure 3).
Figure 3: Multimorbidity network of migraine in age group of >72 years adjusted for both sexes showing the complex interaction among different diseases. This seem to as the age is progressed the comorbidity network of migraine expand exponentially (Morbinet).
Females with complex multimorbidity networks have several female dominance risk factors, one of which is the existence of female dominance estrogen hormone, and it has also been well established that ESR1 and its polymorphisms have a significant influence on illness risk by increasing disease likelihood.
FUTURE PERSPECTIVE
fter discussing various aspects of the condition, the interesting and necessary question is what the condition’s future is, and what are the current possibilities for understanding the condition?
Machine Learning in the diagnosis of headache
Identifying a disease is an important stage in the treatment process and takes a lot of time and effort to identify a disease (i.e., disease diagnosis). Humans are prone to making mistakes that go unnoticed, making it difficult to assess the type and severity of the condition. As a result, different techniques have been developed to assess this human aid diagnostic error and one such astonishing method is artificial intelligence” (AI). With little human interaction, computer intelligence is used to replace a doctor’s diagnostic skills in a variety of healthcare applications [85]. Machine learning (ML), is a branch of artificial intelligence focused on the creation and development of algorithms that enable computers to evolve behaviors based on empirical data. It has become increasingly popular in recent years and has had an impact on many fields [86] with a recognizable success for improving medical diagnostics by increasing accuracy, reproducibility, and speed, as well as reducing clinician burdens [87].
Concerning headaches, ML appears to be a promising platform for creating intelligent solutions because it expands the range of analytical operations required to boost diagnostic proficiency for primary headache diagnosis [88,89] . It may also have the potential to help identify the best predictor responsible for the progression of the disease [90].To diagnose, ML requires a dataset that is readily available online (act as a secondary source) or directly from patients (primary source), giving a doctor the advantage, they need to make a precise diagnosis. However, it is well known that the headache dataset contains a significant number of features such as somatosensory evoked potential, pain, analgesics, score MDQ-H (Medication-dependence questionnaire in headache), total pain month and examine patient-reported surveys, etc. [91]which showed high dimensionality, therefore necessitating the use of a different machine learning algorithm. A few examples of algorithm includes Random Forest (RF), Extreme Gradient-Boosting Trees (EGBT), K-nearest neighbors, multilayer perceptron, linear discriminant analysis, logistic regression [92],
Convolutional Neural Network (CNN) [93], Naive Bayes classifier, Support Vector Machine classifier (SVM) [94] that have been used for Computer-Aided Classification (CAC).
Using such discriminative features, these classification algorithms helped in classifying the migraine as migraine or not migraine, chronic or not chronic, and so on, increasing the chance of getting a high probability of correctly classifying the subject. Using a classification algorithm that makes extensive use of data from a variety of functional and structural MRI patterns, patients with primary headaches can be precisely separated from a group of controls [95].
Enclosing the section, ML has been a promising tool for computer-based diagnosis, finding the best predictor for disease occurrence, etc. But many things have yet to be used, one of which is the use of machine learning on the classification of migraine subtypes using genetics/gene variants as a feature, and the second is the future prediction for the outcome of comorbid conditions. Also, by combining the applications of machine learning techniques, neuronal network models, imaging technology, and clinical data, researchers anticipate being able to develop trustworthy biomarkers that will enable them to choose the best treatment for each headache patient [96].
Drugs and migraine
Different medicines have been available for treatment but all medicines are symptomatic medicines therefore, no cures are available [97]. Migraine treatment is divided into three categories: preventative, abortive, and biofeedback. Existing therapies are frequently ineffective in relieving pain, causing side effects, and increasing the risk of medication-related headaches. However, recent research has revealed a promising and potential treatment option that includes atogepant [98,99], erenumab [100, 101], rimegepant [102] and galcanezumab, and fremanezumab. Eletriptan may be the best treatment for migraines overall, according to a network meta-analysis. In the meantime, ibuprofen’s high level of tolerance makes it a viable alternative [103]. Another recent network meta-analysis confirmed that CGRP mAbs, particularly galcanezumab 240 mg, monthly fremanezumab, and eptinezumab 300 mg, are the most effective treatment for patients with migraines who have not responded to prior therapies [104]. Prescription of these CGRP inhibitor medications may enhance the quality of life for migraine sufferers. Pathology and altered vulnerability to disease arise from alterations in certain proteins and the signaling pathways they regulate. Therefore, relying on existing medications may not deliver a complete cure, so this opens the possibility for the discovery of novel therapeutics.
Here are a few examples of newly-emerging potential migraine therapies that go beyond anti-CGRP [105] and include acid-sensing ion channels [106], Short-Chain Fatty Acids (SCFAs) [107], PAC1 receptor [108], KATP channel blockers [109], use of nanoparticle for the drug delivery [110], prevention of chronic migraine by blocking microglia P2X4R-BDNF signaling pathway [111], BKCa channel [112], G-protein coupled receptors, glutamate, ion channels, and neuro-modulatory devices see review [113]. These new potential candidate drugs may prove to be a wonderful, safe anti-migraine medication and, hopefully, will have a good impact on the lives of migraine sufferers.
Strengths and limitations of the study
Regarding the strength of the present review, different aspect of migraine which includes migraine as an inflammatory disorder, migraine as a channelopathy, and such abrupt phenotypes are due to the alteration in the genes, epigenetics dysregulation of different hemostatic pathways which leads to the decreased pain threshold susceptibility and interestingly the environmental risk factors hinder the with the susceptibility. Also, we have discussed the role of machine learning in computer aid diagnoses and how it will revolutionize the face of diagnosis of migraine. In addition to some limitations, there is also the substantial contribution of vascular change in the genesis of the migraine [153], which we have not explored in this review due to space constraints. Therefore, this concluding conviction, with specific limitations, is incapable of capturing all of the parts of migraine causation due to their multifaceted nature and interplay, and so only represents the “tip of the iceberg,” leaving the remainder undiscovered.
CONCLUSION
Migraine is a complicated neurological disorder characterized by unusually low neuronal hyper excitability, referred to as “migrainous brain,” which is caused by mutations in genes involved in cellular hemostatic maintenance. Environmental influences, on the other hand, and their intricate interplay with the migrainous brain, obstruct the normal cellular system and induce pain (the cardinal character). The repeated disruption damages the normal brain structure and leads to numerous brain lesions that may become candidate structures for late onset neurological diseases. To this end, it can’t be said that migraine is not just a headache but more than it, As migraine is an intricate and complicated condition, its complexity cannot be fully characterized in a single article, which is why it is beyond the scope of this piece of article, and this merely represents only the “tip of the iceberg”
Availability of data and material
All the data related to the review are present in the manuscript.
AUTHOR’S CONTRIBUTION
Detail of the author’s contribution, according to the CRediT (Contributor Roles Taxonomy) System: Parvinder Kumar & Amrit Sudershan contributed to the stud design, Amrit Sudershan, Meenakshi Bhagat, & Shikha Bharti downloaded & filter the literature, Amrit Sudershan drafted the manuscript, picture drawing and table editing, Parvinder Kumar, Sheetal Bhagat & Pallavi Sachdeva edited the manuscript, Parvinder Kumar finalize the manuscript.
REFERENCES
1. Raouf R, Quick K, Wood JN. Pain as a channelopathy. J Clin Invest. 2010; 120: 3745-3752.
13. Leao AAP. SPREADING DEPRESSION OF ACTIVITY IN THE CEREBRAL CORTEX. J Neurophysiol. 1944; 7: 359-390.
26. Felsenfeld G. A brief history of epigenetics. Cold Spring Harb Perspect Biol. 2014; 6: a018200.
27. Weinhold B. Epigenetics: the science of change. Environ Health Perspect. 2006; 114: A160-167.
31. Zakhari S. Alcohol metabolism and epigenetics changes. Alcohol Res. 2013; 35: 6-16.
47. Muller WA. Getting leukocytes to the site of inflammation. Vet Pathol. 2013; 50: 7-22.
50. Bartley J. Could glial activation be a factor in migraine? Med Hypotheses. 2009; 72: 255-257.
53. Pietrobon, D. Neuronal calcium channels and migraine. Biophysical Journal. 2009; 96: 202a–203a.
59. Gordon N. Episodic ataxia and channelopathies. Brain Dev. 1998; 20: 9-13.
62. Dworakowska B, Do?owy K. Ion channels-related diseases. Acta Biochim Pol. 2000; 47: 685-703.
68. Schürks M, Winter AC, Berger K, Buring JE, Kurth T. Migraine and restless legs syndrome in women. Cephalalgia. 2012; 32: 382-389.
140. García-Martín E, Esguevillas G, Serrador M, Alonso-Navarro H, Navacerrada F, Amo G, García-Albea E, et al. Gamma-aminobutyric acid (GABA) receptors GABRA4, GABRE, and GABRQ gene polymorphisms and risk for migraine. J Neural Transm (Vienna). 2018; 125: 689-698.
141. Deng Y, Huang J, Zhang H, Zhu X, Gong Q. Association of expression of DRD2 rs1800497 polymorphism with migraine risk in Han Chinese individuals. J Pain Res. 2018; 11: 763-769.
145. Chen M, Tang W, Hou L, Liu R, Dong Z, Han X, et al. Tumor Necrosis Factor (TNF) -308G>A, Nitric Oxide Synthase 3 (NOS3) +894G>T Polymorphisms and Migraine Risk: A Meta-Analysis. PLoS One. 2015; 10: e0129372.
148. Sudershan A, Bhagat M, Singh K, Pushap AC, Kumar H, & Kumar P. A Comprehensive Investigation of Risk Association Between the -786 T > C, + 884 G > A, VNTR, rs743506, rs3918226 of eNOS and Susceptibility of Migraine: A Updated Meta-Analysis Utilizing Trial Sequential Analysis. Journal of molecular neuroscience.
150. Sudershan A, Pushap AC, Bhagat M, Sharma I, Kumar H, Digra SK, Kumar P. (2023). Comprehensive analysis of genes associated with migraine in the Indian population: a meta-analysis of genetic association studies with trial sequential analysis. Sci Rep. 2023; 13: 19070.
151. Ashina M. Vascular changes have a primary role in migraine. Cephalalgia. 2012; 32: 428–430.