Effects of a Novel Nail Lacquer Based on Hydroxypropyl-Chitosan (HPCH) in Subjects with Fingernail Onychoschizia
- 1. DermIng, Clinical Research and Bioengineering Institute, Italy
- 2. Scientific Department, Polichem SA, Switzerland
Abstract
HPCH nail lacquer is a medical device intended to relieve symptoms and signs of nail dystrophy. Its main ingredients are hydroxypropyl-chitosan (HPCH), Equisetum arvense and methylsulphonyl-methane. In order to investigate the exact role of HPCH in this medical device, the HPCH nail lacquer was compared to another nail lacquer (P-09-005) with identical composition, except for the presence of insoluble chitosan instead of HPCH. Thirty-four healthy women with onychoschizia of the fingernails were included. Both products were applied by all subjects once daily on the affected fingernails of either hand at random, for 4 weeks. The severity of nail signs was assessed using a 0-3 scale. Nail surface profilometry was assessed by morphometrical analysis of nail grooves on nail casts. Visual score of onychoschizia improved at T4 in 74% of volunteers with HPCH nail lacquer and in 52% with P-09-005 (Wilcoxon test p<0.05 between treatments). Severe onychoschizia, present in 35% of patients at baseline, improved at T4 in 80% of subjects with HPCH nail lacquer (Dunnett test p<0.05: T4 vs T0) and in 42% with P-09-005. On the morphometrical analysis a significant reduction of rugosity of the longitudinal nail grooves was noticed (Dunnett test p<0.05: T4 vs T0): 19% for HPCH nail lacquer and 16% for P-09-005 (not significant between treatments). Both products were well tolerated. In conclusion, HPCH nail lacquer proved to be effective in improving the nail structure and appearance in subjects with onychodystrophy. The presence of HPCH in the formulation was specifically effective in decreasing lamellar splitting.
Keywords
Equisetum arvense , Hydroxypropyl-Chitosan (HPCH) , Nail lacquer , Onychodystrophy , Onychoschizia
Citation
Sparavigna A, Caserini M, Tenconi B, De Ponti I, Palmieri R (2014) Effects of a Novel Nail Lacquer Based on Hydroxypropyl-Chitosan (HPCH) in Subjects with Fingernail Onychoschizia. J Dermatolog Clin Res 2(2): 1013.
ABBREVIATIONS
HPCH: Hydroxypropyl-Chitosan; MSM: Methyl Sulfonyl Methane
INTRODUCTION
Brittle nail is a common disorder affecting about 20% of the population and women are twice as frequently affected as men. Moreover, it often occurs in elderly people due to the slow nail growth at that age, but not only, as young ladies may often be affected [1].
Brittle nails are characterized by roughness of the surface of the nail plate and fragility. They are mostly associated with an abnormality of keratin, keratin associated proteins, water and/ or lipid content. Patients with brittle nails usually complain that their nails are soft, dry, weak, easily breakable and unable to grow long. These alterations can be caused by skin disease such as psoriasis or fungal infections, unbalanced diet, metabolism disorders, continuous use of water or chemical agents such as solvents and detergents, traumas of the nail plate or inadequate footwear in case of toenails. The more common clinical signs are pathobiological changes to the nail plate horizontal layering (onychoschizia) and to the nail matrix-longitudinal ridging and splitting of the nails (onychorrhexis) [1].
Several systemic and topical therapies for the treatment of brittle nails have been tried. Over the years, calcium, gelatin, multivitamins, silicon and tazarotene cream have been used with little efficacy or mixed results [1,2].
HPCH nail lacquer is a medical device based on a new technology that has recently entered the markets. It is a nail lacquer specifically developed to restructure and remineralise nails affected by malformation, fragility and local pain. The new technology is based on hydroalcoholic solutions of hydroxypropyl-chitosan (HPCH), a water soluble semisynthetic derivative of chitosan which acts as a film forming agent. HPCH dissolves in high percentage in water, has affinity with air, is highly plastic and forms a highly elastic, smooth and almost invisible film. HPCH increases the dispersion of other ingredients, acting as a carrier at the nail level, by employing chitin derived hydrosoluble amino-polysaccharides. Chitosan and its derivatives possess adhesive properties towards different biological tissues due to their positive charge. Moreover, the free hydroxypropyl groups of HPCH may interact with keratin, by hydrogen bonding and other weak interactions that could contribute to the improved drug transport and release [3,4]. The correct level of hydration is important for the nail, which becomes dry and brittle if it does not contain enough water. The other two HPCH nail lacquer ingredients are horsetail (Equisetum arvense) and methyl sulfonyl methane (MSM). Horsetail has been used in different indications since the 12th century in various European countries [5,6]. Among all herbs, horsetail contains the highest amount of organic silica, which binds to keratin, remineralising and restructuring the nails.
Methylsulfonylmethane (MSM) is an important source of sulfur found in the human diet. MSM is found in foods, including fruit, tomatoes, tea, and coffee; in human and bovine milk and in human urine. MSM contains 34% elemental sulfur [7].
Sulfur is a therapeutic agent useful in a variety of dermatologic disorders. Its keratolytic action is due to formation of hydrogen sulfide through a reaction that depends upon direct interaction between sulfur particles and keratinocytes [8].
The sulphur contained in MSM is taken up by nail keratin, thus strengthening the nail plate.
In order to investigate the exact role of HPCH in the medical device, the aim of the present study was to evaluate the effects of the water soluble HPCH nail lacquer versus those of another lacquer (P-09-005) with identical composition, except for the presence of insoluble chitosan instead of HPCH. The comparison was performed on the fingernails of subjects with onychoschizia by means of clinical and non-invasive instrumental evaluations.
MATERIALS AND METHODS
Materials
The two nail lacquers used in this study, manufacturer Polichem S.A. Lugano, were identical except for the film forming agent which was hydroxypropyl-chitosan (water soluble) in HPCH nail lacquer and chitosan (water insoluble) in P-09- 005. The other ingredients were: E. arvense glycolic extract, methylsulfonyl methane, diethylene glycole monoethylether, water, ethanol (Table 1).
Table 1: Qualitative composition of HPCH nail lacquer (A) and P-09-005 (B).
| (A) |
| HPCH nail lacquer |
| Equisetum Arvense (Horsetail) |
| Methylsulfonal Methane |
| HPCH |
| Ethyl alcohol 99.9° |
| Diethylenglycol monoethylether |
| Water |
| (B) |
| P-09-005 |
| Equisetum Arvense (Horsetail) |
| Methylsulfonal Methane |
| Chitosan |
| Acetic Acid |
| Ethyl alcohol 96° |
| Diethylenglycol monoethylether |
| Water |
Study design
The study was an open label, randomized, single center, within subjects comparison between the two nail lacquers. Thirty-four healthy women, aged between 20 and 70 years (mean 46 yrs), with fingernail onychoschizia due to exogenous causes such as chemical agents and/or trauma, were included in the study after providing their written informed consent. Patients having concomitant clinical conditions like onychomycosis, nail psoriasis or bacterial infections were excluded by the trial.
The clinical signs evaluated at baseline and followed during the trial were: dystrophy, onycholysis, onychoschizia and nail fragility. Each volunteer applied HPCH nail lacquer to all fingernails of one hand, randomly selected according to a randomization list, while the fingernails of the controlateral hand were treated with the comparator (P-09-005). All applications were performed in the evening at bedtime for an average of 4 weeks.
Starting from the second application the subject was required to remove the previous nail lacquer before each new application, by simply hand washing with neutral soap for HPCH medical device and by means of a cotton flock soaked in ethanol for P-09- 005.
Assessments
The efficacy assessments were performed at baseline (T0), after two weeks of treatment (T2) and after four weeks of treatment (T4). At each visit the clinical signs were evaluated by means of a four-point scale where 0=absent, 1=light, 2=moderate, 3=severe. Moreover an instrumental assessment of the nail surface alterations was done by means of optical profilometry. Both clinical and instrumental assessments were performed by the Investigator who treated the subjects.
Casts (silicon replica) of nail lamina using silicone rubber were taken at T0, T2 and T4 from the thumb of both hands after removal of the products and analysed by a computerized image analyzer in order to quantify nail surface alterations at baseline and any changes due to the treatment according to a previously published method [9]. Silicon replicas were taken under standard conditions in order to be reproduced at any time. Pictures of silicon replicas at a light angle of 45° revealed shadows on the replica (which is the negative image of longitudinal and transverse grooves in the nail lamina). These shadows were transformed into a grey scale whose intensity was proportional to the intensity of the shadows and therefore to the depth of the grooves. The surface of the replica underwent a scanning procedure highlighting any alterations in the longitudinal and transverse grooves (“Beau Lines”) in the nail. The scans were taken almost at the centre of basal replica, and always at the same level but not at the same preceding height (since the nail plate had grown) for the previously mentioned observations times. The precision in the measurement is obtained through the morphology of the nail plate and by identifying precise traces to be used as reference. The mean roughness (Ra) was calculated as the arithmetical mean of all deviations in absolute values [9].
Statistics
Evaluation of each study product versus baseline: the clinical evaluation results were analyzed by the Friedmann test, followed by Dunnett’s test in case of statistically significance; the instrumental evaluations were analyzed by ANOVA followed by Dunnett’s test in the case of statistical significance.
Comparison of the two test products over time: the Wilcoxon test was used for the clinical evaluations and the Student’s t test for the instrumental evaluations.
RESULTS AND DISCUSSION
The clinical signs observed at baseline in all 34 women were onychoschizia and nail fragility, while seven and five subjects presented dystrophy and onycholysis, respectively, although in a light form. The exogenous causes of nail disorders are summarised in the table 2.
Table 2: Exogenous causes of nail disorders at baseline.
| EXOGENOUS CAUSES | SUBJECTS% |
| Chemical agents | 6% |
| Trauma | 18% |
| Humidity | 9% |
| Other (constitutional, dietary factors, hobbies, gloves use) | 32% |
| Trauma + Humidity | 14% |
| Chemical agents + Humidity | 18% |
| Chemical agents + Other | 3% |
As shown in Figure 1,
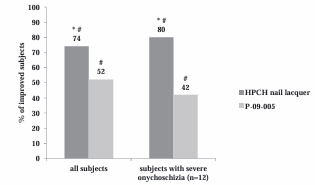
Figure 1 Proportion of patients with clinical improvement of onychoschizia after 4 weeks of treatment (PP population and sub-group of subjects with severe onychoshizia at baseline). HPCH nail lacquer vs. P-09-005 = * p values < 0.05 (Wilcoxon test). T4 vs. T0 = # p values < 0.05 (Dunnett’s test)
a significant reduction in onychoschizia was shown by both products after a 4 week treatment (Dunnett’s test: p<0.05 T4 vs T0). At the end of treatment (T4) the visual score improved in 74% of the subjects treated with HPCH nail lacquer and in 52% of those treated with P-09-005, the difference between the two groups being statistically significant (Wilcoxon test, p<0.05). In the sub-group of patients with severe onychoschizia at baseline, equal to 35% of the enrolled subjects, the improvement vs. baseline was 80% for HPCH medical device (p<0.05) and 42% for P-09-005 (not statistically significant). Figure 2 and 3 show representative pictures of a woman with a nail treated with HPCH nail lacquer (Figure 2)

Figure 2 Onychoschizia completely resolved in a fingernail treated with the HPCH nail lacquer (b) compared to baseline (a) after 4 weeks of treatment.
compared to the controlateral nail treated with the lacquer containing the insoluble chitosan (Figure 3)

Figure 3 Onychoschizia still present in a fingernail treated with the insoluble chitosan nail lacquer (b) compared to baseline (a) after 4 weeks of treatment
at T0 and T4. As shown in Figure 4 nail fragility,

Figure 4 Proportion of patients with clinical improvement of nail fragility after 2 and 4 weeks of treatment (PP population). T2 and T4 vs. T0 = # p values < 0.05 (Dunnett’s test)
also present in all subjects at baseline, improved after 2 weeks of treatment in 45% of volunteers in both groups and after 4 weeks in 81% of subjects treated with HPCH nail lacquer and in 74% of those treated with P-09-005 (Dunnett test, p<0.05 T2 vs T0 and T4 vs T0 for both products), but the difference between the two lacquers was not statistically significant.
As shown in Figure 5,
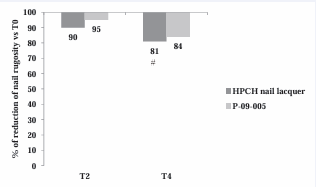
Figure 5 Proportion of patients with clinical improvement of nail rugosity by morphometrical analysis after 2 and 4 weeks of treatment (PP population). T4 vs. T0 = # p values < 0.05 (Dunnett’s test)
the optical profilometry of the longitudinal nail grooves revealed a significant reduction (Dunnett test, p<0.05 T4 vs T0) of the Ra parameter, representing the arithmetic average of nail rugosity, with both HPCH nail lacquer (19%) and P-09-005 (16%). No statistically significant difference was found between groups either at T2 or at T4. No change was noticed in transverse grooves. As regards dystrophy and onycholysis, present in 21 and 15% of women at baseline, respectively, no variations were found at the end of the study.
The safety of the lacquers was judged to be good or excellent by both volunteers and the investigator. Also cosmetic acceptability of both lacquers was found to be good by the volunteers.
As Rigopoulos and Daniel state in a recent review [1], treatment of brittle nails is never easy. The identification of causative factors may be immediate for exogenous factors, such as occupational exposure to chemicals, but is more complicated for metabolic and nutritional disorders, which in some cases are they cause of nail brittleness in patients. Several systemic therapies for the treatment of brittle nails have been tried. Over the years, calcium, gelatin, multivitamins, etc., have been used with low efficacy. Silicon has recently been advocated with mixed results [10] as has tazarotene cream 0.1% [11].
Previous clinical studies showed that HPCH nail lacquer, when applied to the fingernails, reduced longitudinal grooves, lamellar splitting and nail fragility in women with nail plate alterations [3]. Other non-clinical and clinical studies have been performed to investigate the potential effects of hydroxypropylchitosan (HPCH), the main ingredient of the new hydro-lacquer technology. HPCH, when applied to the nails, forms a highly elastic, smooth and almost invisible film that adheres to the nail structures, protecting them against physical and microbiological injuries [4]. In-fact, HPCH is a chitosan derivative that has the great advantage to be soluble in cold water without any pH correction, due to the fact that the chitosan polymer backbone bears hydrophilic residues. These residues are believed to be the basis of the observed high affinity of HPCH with keratin, a mechanism in its turn responsible for the improved restructuring process of the nail plate.
Moreover, a clinical study was performed in adult patients with mild-moderate nail psoriasis of the matrix and/or the nail bed. The nail lacquer was applied o.d. on the affected fingernails of the left hand for 24 consecutive weeks, while the right hand was used as control. At the end of treatment, patients showed a 63-72% reduction of pitting, leukonychia, onycholysis and “Nail Psoriasis Severity Index” score. The treatment evaluation was classified as very satisfying or good by 79% of patients [12]. All mentioned studies clearly showed that the HPCH nail lacquer is effective in patients with nail plate alterations, including psoriatic onychodystrophy. The efficacy was also confirmed by Rigopoulos and Daniel [1] who reported the HPCH nail lacquer, recently approved as medical device by FDA in the United States, to be effective in 50% of patients (8 out of 16 treated subjects) with brittle nails after only 8 weeks of treatment.
This clinical study adds new information regarding the properties of the HPCH nail lacquer, after investigating its effects when compared to an identical lacquer containing an insoluble chitosan instead of HPCH. After 4 weeks of treatment both products determined important and statistically significant reductions in the main nail disorders (in particular onychoschizia and nail fragility) and revealed a good smoothing action. HPCH nail lacquer was generally more active than P-09-005. In particular, the superiority of the product was evident in reducing the visual scores of onychoschizia. In the subgroup with severe onychoschizia, the clinical sign improved in the 80% of the treated subjects (p<0.05, T4 vs T0) with HPCH medical device, but only 42% (not statistically significant) with P-09-005. Thus, the superiority of HPCH nail lacquer versus P-09-005 clearly demonstrates that HPCH plays a positive role in the treatment of nail disorders. The results obtained are valid in the limit of the experiment done.
CONCLUSION
In conclusion, the results of the present study indicate that the new HPCH nail lacquer, when applied on the nails, is effective in improving the nail structure and appearance in subjects with onychodystrophy. The efficacy of the HPCH nail lacquer is specifically due to the presence of water soluble HPCH. In-fact the study proves that a nail lacquer, containing a water insoluble chitosan, is less effective than the HPCH nail lacquer in improving clinical signs of onychoschizia.
CONFLICT OF INTEREST
Maurizio Caserini and Renata Palmieri are employees of Polichem S.A.
Polichem S.A. sponsored all phases of the study and supplied the trial product.
REFERENCES
- Dimitris R, Ralph D. Management of simple brittle nails. Dermatol Ther. 2012; 25: 569-573.
- Draelos ZD. Understanding and treating brittle nails. Cosmet Dermatol. 2009; 22: 598–599.
- Sparavigna A, Setaro M, Genet M, Frisenda L. Equisetum Arvense in a new transungual technology improves nail structure and appearance. J Plastic Dermatol. 2006; 2: 31-38.
- Sparavigna A, Setaro M, Frisenda L. Physical and microbiological properties of a new nail protective medical device. J Plastic Dermatol. 2008; 4:1.
- Madaus G. Equisetum arvense et Equisetum hiemale. In: Madaus G: Lehrbuch der biologischen Heilmittel. Georg Thieme Verlag Leipzig. 1938; 1: 1267-78.
- Hiermann A. Equisetum. In: Blaschek W, Ebel S, Hackenthal E, Holzgrabe U, Keller K, Reichling J (eds.): Hagers Handbuch der Drogen und Arzneistoffe. Hager ROM 2006; Springer electronic media.
- Parcell S. Sulfur in human nutrition and applications in medicine. Altern Med Rev. 2002; 7: 22-44.
- Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988; 18: 553-558.
- Baran R, Sparavigna A, Setaro M, Mailland F. Computerized image analysis of nails affected by fungal infection: evaluation using digital photographs and manually defined areas. J Drugs Dermatol. 2004; 3: 489-94.
- Haneke E. Onychocosmeceuticals. J Cosmet Dermatol. 2006; 5: 95-100.
- Sherber NS, Hoch AM, Coppola CA, Carter EL, Chang HL, Barsanti FR, et al. Efficacy and safety study of tazarotene cream 0.1% for the treatment of brittle nail syndrome. Cutis. 2011; 87: 96-103.
- Cantoresi F, Sorgi P, Arcese A, Bidoli A, Bruni F, Carnevale C, et al. Improvement of psoriatic onychodystrophy by a water-soluble nail lacquer. J Eur Acad Dermatol Venereol. 2009; 23: 832-834.








































































































































