Topical Treatment of Tocotrienol-Rich Fraction (TRF) on Deep Partial-Thickness Burn Wounds in Rats
- 1. Department of Bioscience, University Putra Malaysia, Malaysia
- 2. Department of Biomedical Science, Universiti Putra Malaysia, Malaysia
- 3. Department of Pathology, University Putra Malaysia, Malaysia
- 4. Department of Nutrition and Dietetics, University Putra Malaysia, Malaysia
Abstract
Many studies have shown that tocotrienol has high antioxidant activity and is protective against brain cell damage and cancer. Tocotrienol-rich fraction (TRF) of palm oil consists of 25% alpha-tocopherol and 75% tocotrienol. We investigated the effect of topical application of a TRF cream formulation on a deep partial-thickness burn model in Sprague-Dawley rats. The assessment was based on the wound contraction rate, clinical evaluation, and histopathological changes. Animals (n = 3 per group) were treated with silver sulfadiazine; cream base; or 3%, 4%, or 5% TRF. Uniform deep partialthickness burns were created on the dorsal of the rats. The TRF treatments accelerated the wound contraction rate, enhanced re-epithelialization, and stimulated granulation tissue formation and the regeneration process. The TRF treatment reduced hyperemia, edema, crusting, and the re-epithelialization period. All of the TRF treatments exhibited similar ability for treating the burn wounds. Our findings indicate that this TRF formulation is effective for treating deep partial-thickness burns. The mechanism of action of TRF on wound healing will be investigated in future studies.
Keywords
Burn wound healing, Rats, Second-degree animal model, Tocotrienol-Rich Fraction (TRF), Vitamin E, Wound contraction
Citation
Zaini AA, Khaza’ai H, Ali RM, Abdul Mutalib MS, Baharuddin AA (2016) Topical Treatment of Tocotrienol-Rich Fraction (TRF) on Deep Partial-Thickness Burn Wounds in Rats. J Dermatolog Clin Res 4(1): 1063.
INTRODUCTION
Most patients admitted to the burn unit have deep partialthickness burns [1] and most of the medications prescribed for burns may cause adverse reactions such as allergy and toxicity [2]. Hence, it is important to find safer and more effective drugs to improve the care processes and outcomes in patients with burns. All burns should be treated immediately to reduce damage to the skin and underlying tissue. Therefore, there is a need to develop a product that can be applied immediately and easily used at home.
Vitamin E appears to be one of the least toxic of fat soluble vitamins and does not cause adverse effects at high doses [3]. Oil from barley, wheat germ, and palm fruits are a crucial source of vitamin E, which is composed of subfamilies that include tocopherols and tocotrienols. Many studies on human skin have focused on the therapeutic effects of vitamin E in the form of tocopherol. While data on the therapeutic effect of vitamin E in tocotrienol form are scarce, accumulating evidence has shown that tocotrienol has higher antioxidant activity than tocopherol [4]. This may be attributed to the three double bonds on the hydrophobic side chain of tocotrienol that render it far more efficient at penetrating adipose, brain, and liver tissue, which has saturated fatty layers [5]. Tocotrienol also has anti-cancer, cardioprotective, and neuroprotective and gastroprotective effects and antidiabetic properties [6].
The accumulating data suggest that, in any type of wounding, reactive oxygen species production is increased and antioxidant levels are lower. Reactive oxygen species cause tissue damage and a lipid peroxidation chain reaction, which will cause more tissue damage if it is uninterrupted. Vitamin E is the most potent lipid-soluble antioxidant and can scavenge the oxygen radicals produced by injury [7].
Vitamin E is used widely in topical applications, such as in cosmetic and skin care products. However, most vitamin E skin care products contain only alpha-tocopherol and a small amount of synthetic alpha-tocopheryl acetate, which requires hydrolyzation during absorption to exhibit its activity [8,9]. Tocotrienol is a potent antioxidant and has anti-inflammatory properties that may aid in improving burn wound healing [10]. To further understand the therapeutic effect of the tocotrienol-rich fraction (TRF) of palm oil on burn wounds, we examined the effect of three concentrations of TRF in a deep partial-thickness burn wound rat model. We evaluated the effect of topical application of TRF on improving the outcome of burn wounds based on wound closure rate, clinical evaluation, and morphological changes of the burn wound tissue in Sprague-Dawley rats.
MATERIALS AND METHODS
Methods
Experimental Animals: A total of 60 male Sprague-Dawley rats aged 6-8 weeks and weighing 180–250 g were used. The rats were acclimatized to laboratory conditions for one week prior to the experiment. The rats were housed in individual cages containing wood shavings that were changed daily and had ad libitum access to a standard pellet diet and tap water. All animals were weighed before the experiment and were handled in accordance with current University Putra Malaysia (UPM) guidelines for the care of laboratory animals and ethical guidelines for investigations.
Study Design: Sixty rats were assigned randomly to five treatments group (n = 7 per group): cream base (negative control); silver sulfadiazine (Silverdin® cream [SSD]; Sunward Pharmaceutical, Johor, Malaysia; positive control), 3%, 4%, and 5%TRF. Each rat from five different treatments was sacrificed on day 0, 7, 14 and 21 of treatment for histology assessment. The design of the animal groups is shown in (Table 1). The Animal Care and Use Committee of the UPM Faculty of Medicine and Health Sciences approved the experimental protocol (Reference No: 00495/03 Dec 2012).
Preparation and Storage of Base Cream and TRF Cream: The base cream formulation containing methyl parabens, cetyl alcohol, butylene alcohol, aminoethyl propanol, and methylparaben was prepared by Dr. Huzwah Khaza’ai of the Department of Biomedical Science, University Putra Malaysia, and was stored in separate commercial cream containers at 4°C.
TRF formulation (Gold Tri E70; Sime Darby, Puchong, Selangor) is a combination of a tocotrienol-rich fraction with the base cream formulation. The calculation for the 200g total formulation, at 3% was as follows; 6g of TRF was added into 194g of base cream into a sterile amber glass bottle with a metal lid. The formulation was stir by using a sterile disposable spatula in a fume hood. The base cream formulation was added to dilute a final concentration of the 3%, 4%, and 5% TRF cream. TRF cream was stored in a commercial cream container at 4°C. The preparation of TRF cream was determined based on the weight
Table 1: Design of animal groups.
| Treatments | Days of sacrificing animals | |||
| Day 0 | Day 7 | Day 14 | Day 21 | |
| Cream base | n=7 | n=7 | n=7 | n=7 |
| 3% TRF | n=7 | n=7 | n=7 | n=7 |
| 4% TRF | n=7 | n=7 | n=7 | n=7 |
| 5% TRF | n=7 | n=7 | n=7 | n=7 |
| Silver Sulfadiazine | n=7 | n=7 | n=7 | n=7 |
of the TRF used, as follows: X g TRF = (X%TRF) × (total volume of the formulation)
Animal Sedation: The rats were anesthetized with single intramuscular injections of xylazine (6 mg/kg Xylazil-20 Ilium Veterinary Product; Troy Laboratories. Pty Ltd, Glendenning, NSW, Australia) and ketamine (85 mg/kg ketamil injection; Troy Laboratories. Pty Ltd, Glendenning, NSW, Australia) using a Terumo 27 G × 0.5-inch needle and Terumo 1-mL syringe (Terumo Corporation, Tokyo, Japan) before their backs were shaved.
Burn Wound Creation: Using the method described by Sarkhail et al., [11] with some modification, deep partialthickness burn wounds were created on the shaved backs of the rats using a 15-mm diameter temperature-regulated soldering iron (single-channel power unit WD 1000 with 80-watt WSP80; Weller, Besigheim, Germany). The soldering iron was heated to 100° C and placed on the back of a rat for 10 s by free fall, without any pressure from the researcher’s hand. The pressure exerted on the skin corresponded to the 20 g mass of the soldering bar used for the burn induction. To confirm the degree of burn injury, the wound tissues were collected by excision using scalpel blade at 5 min post-burn and used to prepare hematoxylin and eosin (H&E)-stained sections for pathological examination. The H&Estained sections did not show any sign of tissue injury (Figure 1),
Figure 1 Microscopic view of H&E stained at 5 min post-burn. No sign of tissue injury.
indicating that at 5 min post-burn, the cells and tissues had not responded to the injury by homeostasis and inflammation. However, H&E-stained sections obtained on day 7 of injury revealed that deep partial-thickness burns, where a thick crust with epidermal and dermal destruction could be observed (Figure 2).
Figure 2 H&E-stained sections on day 7 of injury revealed a deep partial-thickness burn with epidermal and dermal destruction.
Treatment Protocol: Prior to the TRF treatment, the wounds were cleaned with a sterile saline solution and were dried using sterile gauze. Treatment was applied 5 min after the burn and was applied once daily for 21 days. Using sterile gloves, all creams were applied evenly in sufficient amounts to cover the entire wound area. Animals were housed in individual cages following the treatment; the wounds were uncovered throughout the experimental period.
Macroscopic Assessment: The animals were examined daily for clinical evaluation and every four days for wound contraction.
• Wound Contraction: On day 1, 5, 9, 13, 17 and 21 postburn, the animals were restrained to minimize movement before their wounds were photographed using a Canon PowerShot SD1100IS Digital Ixus 80IS digital camera (Tokyo, Japan) held directly above the wound and with the lens parallel to the plane of the wound surface; consistent lighting was used. Wound contraction was monitored by digital caliper measurement of the progressive changes in the raw wound area. The measurements obtained were set as a scale to allow calculation of the wound dimensions in ImageJ (Software 1.48q, Rayne Rasband, National Institutes of Health, USA). The measurement for each photograph was calibrated using the digital caliper measurements. The wound boundary was traced from the image using a pointer, and then the wound area was calculated using ImageJ. Using the wound area on day 1 as the initial size of the wound, the degree of wound healing was calculated as follows:
Percentage of wound healing (%) = 1 − [wound area on a corresponding day (cm2 )/wound area on day 1 (cm2 )] × 100
• Clinical Evaluation: Wounds were evaluated according to the presence of edema, hyperemia, period of reepithelialization, crusting, scar tissue, and exudate.
• Gross Appearance of the Wound: Digital images of each animal were captured at day 1, 7, 14 and 21 postburn to monitor the general appearance of the wounds.
Microscopic Assessment:
• Skin Sample Collection: Rats were sacrificed at 7-day intervals post-burn to dynamically track the wound healing process microscopically. After day 0, 7, 14 and 21 of each treatment, seven rats from each group of treatment were sacrificed using an overdose of anesthesia, and samples of the wound area were collected. The samples were washed with normal saline to remove debris and were immediately preserved in 10% buffered formalin for at least 72 h at room temperature.
• Histological Examination: Tissue samples were inserted individually into a cassette and labeled accordingly and were then inserted into an Automatic Tissue Processor LEICA TP1020 (Singapore) for 24 h. Following the completion of tissue processing, the samples were embedded in paraffin wax and cooled at 0°C prior to tissue sectioning using an LEICA EG 1160 Tissue Embedding System. Sections (~3–4-μm thick) were obtained using an LEICA RM 2135 microtome and placed in a 40°C water bath. The best slices were selected and fixed onto slides, which were then labeled accordingly.
The samples were placed in a Tissue-Tek Prisma Stainer (Sakura Finetek U.S.A., Inc.; California, United State) for H&E staining, and mounted using DePex. The sections were then assessed and photographed at ×10 and ×40 magnification using a LEICA DM2500 microscope and LAS 4.0 software (Leica Microsystems AG; Wetzlar, Germany). The H&E-stained sections were examined for re-epithelialization, granulation tissue formation, inflammatory cell infiltration, angiogenesis, and skin appendages.
• Histological Scoring Assessment: An experienced pathologist who was unaware of the treatment conditions performed the histological scoring assessment. The stained slides were observed for re-epithelialization, granulation tissue formation, inflammatory cell infiltration, and angiogenesis. The histopathological finding scoring was categorized as listed in (Table 2), which is based on the descriptions by Mehrabani et al., [12].
|
Table 2: Scoring system for histological changes in burn wound healing [12].
|
Statistical Analysis
The results are expressed as mean ± standard error (SE). The group means were compared using 2-way analysis of variance followed by Tukey’s test. Data analysis was performed using GraphPad Prism 6 (GraphPad Software, Inc.; California, United State). A P-value ≤ 0.05 was considered statistically significant.
RESULTS
Wound Contraction
The percentage of wound contraction in the TRF- and SSDtreated groups was statistically significant compared to the negative control group on day 5, 9, and 13 (P < 0.05) (Figure 3, Table 3).
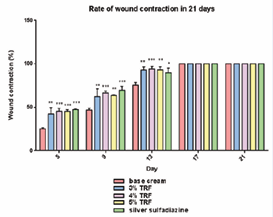
Figure 3 The percentage of wound contraction in the control and treated groups on day 5th, 9th, 13th, 17th and 2st of the deep partial- thickness burn in male Sprague dawley. n = 7.
Table 3: The percentage of wound contraction of the control and TRF- and SSD-treated groups at day 5, 9, 13, 17, and 21 post-burn.
| Treatment | Wound contraction (%) | ||||
| Day 5 | Day 9 | Day 13 | Day 17 | Day 21 | |
| Cream base | 25.29±1.13 | 46.76±2.10 | 75.32±3.28 | 100.00±0.00 | 100.00±0.00 |
| 3% TRF | 42.39±7.21** | 62.38±8.82** | 93.12±3.59** | 100.00±0.00 | 100.00±0.00 |
| 4% TRF | 45.48±3.18*** | 66.46±2.30*** | 94.11±3.11*** | 100.00±0.00 | 100.00±0.00 |
| 5% TRF | 45.40±1.80*** | 63.58±0.70** | 86.18±4.01** | 100.00±0.00 | 100.00±0.00 |
| SSD | 47.35±0.52*** | 69.60±4.47*** | 89.91±5.09* | 100.00±0.00 | 100.00±0.00 |
| Results presented are the mean ± SE. *P < 0.05 compared with the control group (cream base). | |||||
On day 17 and 21 post-burn, there were no significant differences (P > 0.05) between the wound area measurements between all groups (Table 3). In the 4% TRF group, the percentage of wound contraction was consistent and highly significant (P ≤ 0.001) on day 5, 9, and 13. Compared to the 3% and 5% TRF groups, rats in the 4% TRF group had a higher rate of wound closure on the stated days.
Clinical Evaluation
The most striking feature of the treated wounds was the shorter period of crusting and re-epithelialization with discreet hyperemia (Table 4);
Table 4: Clinical evaluation of the wounds based on presence of edema, hyperemia, period of re-epithelialization, and crusting.
| Clinical evaluation | Treatment | ||||
| Cream base | 3% TRF | 4% TRF | 5% TRF | SSD | |
| Edema | Day 1–3 | Day 1–3 | Day 1–3 | Day 1–3 | Day 1–3 |
| Hyperemia | Day 1–12 | Day 3–11 | Day 3–11 | Day 3–11 | Day 3–11 |
| Re-epithelialization | Day 15 | Day 13 | Day 13 | Day 13 | Day 13 |
| Crust | Day 3–15 | Day 3–6 | Day 3–9 | Day 3–11 | Day 3–14 |
no exudate was observed. There was mild edema in all groups until day 3 post-burn. On day 12 post-burn, there was hyperemia and a large amount of crust surrounding the wound margins in the negative control group. However, the treated wounds not only had no crust, but also no secretion or hyperemia. On day 13 after injury, there was complete reepithelialization in all treated groups, in which the wound borders were lined with hair follicles. In comparison, the wounds in the negative control group were covered with an intact crust, and complete re-epithelialization had not yet occurred. Complications or death were not observed during the experimental period
Gross Appearance of the Wound
The wounds appeared white on day 1 post-burn where the rats were in pain with slight edema persisted up until day 3 postburn in all groups. During week 1 post-burn, the wounds in all TRF- and SSD-treated groups were moist, a supple yellow-brown crust had formed, and there was indefinite hair growth around the red-rimmed lesion. By contrast, the wounds in the negative control group were covered with a dry, hard, dark-brown crust, and there was no hair growth (Table 4). Towards the end of week 2 post-burn, the crust had fallen off the wounds in the TRF- and SSD-treated groups and the wounds appeared as moist, soft, yellow-brown lesions; the wounds of the negative control group remained covered with dry, intact, dark brown scabs. The crust falling off indicated complete re-epithelialization in all TRF- and SSD-treated groups. However, the negative control group still had not achieved complete re-epithelialization at this point. The best healing was seen in 3% TRF–treated wounds at 21 days post-burn, in which smaller and linear-shaped scars indicated enhanced wound contraction.
Skin Histology
(Figures 4–6) depict the histology of the biopsied wound tissue on day 7, 14, and 21 post-burn, respectively.
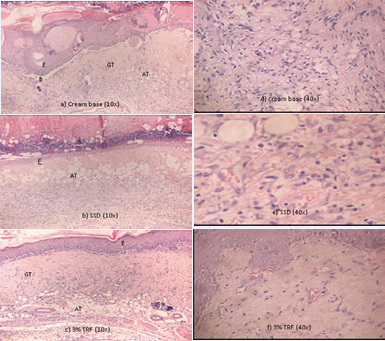
Figure 4 Microscopic view of the histological sections at day 7th of post-burn stained with H&E. Note the advanced regeneration and complete organization of epidermal in the wound treated with (c) 3% TRF in contrast to the delay epidermal regeneration in (a) cream base and (b) SSD group indicating by the presence of crusting. Meanwhile in cream base group the blistering persists, an indication of delayed epidermal repair. Dermal proliferation was delayed in (a) cream base and (b) SSD group as compared to the (c) 3% TRF, indicated by the presence of adipose tissue substitution. In (d) cream base and (e) SSD group, marked inflammatory response still persists. (AT: Adipose Tissue; B: Blistering; E: Epidermis; GT: Granulation Tissue)
At day 7 of injury, epidermal regeneration was observed in all wounds (Figure 4). The TRF-treated wounds exhibited better reepithelialization than that of the control groups. Firstly, the TRFtreated groups had a complete epidermal organization in contrast to the incomplete epidermal organization in 40% of the tissue in the control groups. In the control groups, we observed only two epithelial cell layers, whereas there were three epithelial cell layers in all TRF-treated groups. Secondly, intraepithelial inflammatory cells, particularly lymphocytes, were absent from all TRF-treated wounds but were present in that of the control groups. Thirdly, no crust formation or blistering was observed in the TRF-treated wounds, but crusting was present in both control groups. Meanwhile, in the cream base group the blistering persisted, an indication of delayed epidermal repair. Histologically, there was moderate inflammation in the control groups, whereas there was only mild inflammation in the TRFtreated groups. The inflammatory cells present in control groups (base cream and SSD) were lymphocytes and monocytes, which were located mainly in the diffuse tissue. Low neutrophils count can be observed in all of the five groups. In the angiogenesis study, the negative control had a score of 3 with only 5–6 vessels observed, while the other groups had scores of 4, indicating >7 vessels within the granulation tissue, which was significantly higher in comparison to the negative control.
gher in comparison to the negative control. In (Figure 5)
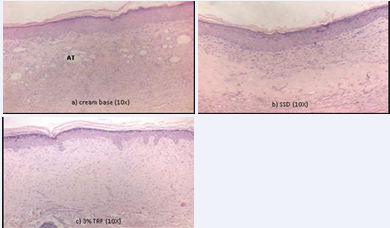
Figure 5 H&E stained at day 14th post-burn showing adipose tissue substitution in (a) cream base. Collagen fibers arrangement in (a) cream base and (b) SSD were thicker and poorly aligned compared to (c) 3%TRF, which indicates a slow fibro proliferative repair. In (c) 3% TRF, less inflammatory infiltration and normal skin morphology was observed based on the normal density and parallel orientation of a collagen fiber. This indicates the skin for 3%TRF points towards the end of the healing process (AT: Adipose Tissue).
(day 14 of injury), proliferation was better in the TRF-treated groups, as indicated by moderate epithelial proliferation in 60% of the tissue, and better granulation tissue formation and collagen matrix organization, as indicated by the absence of adipose tissue substitution and regular as compared to the control groups. In all TRF-treated groups, collagen fiber deposition was healthy and regularly arranged, while collagen fibers in both control groups were thicker and their arrangement was disordered.
In (Figure 6)
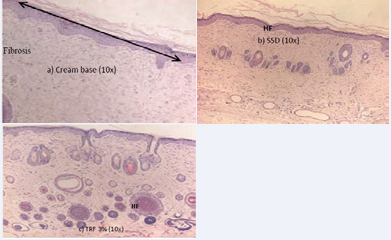
Figure 6 H&E stained at day 21st post-burn showing scar skin in (a) cream base group. In (b) SSD group, H&E stained showed a normal skin with only immature hair follicle. In (c) 3% TRF group, H&E stained showed a normal skin with a mixture of immature and mature hair follicle. (HF: Hair Follicle).
(day 21 of injury), there was dermal papillary layer remodeling and complete epidermal remodeling in the TRF-treated groups. There were a thick granulation layer and well-formed collagen matrix in 60% of the tissue in the SSD- and TRF-treated groups. The collagen fibers in the SSD-treated group were oriented parallel to the epidermis, a characteristic similar to scar formation. There was complete epidermal remodeling in all groups. There were mature and immature hair follicles in the TRF-treated wounds while only immature hair follicles were observed in the SSD-treated wounds. No hair follicles were observed in the cream base–treated wounds. All treatments had a score of 4, indicating complete tissue organization in 80% of tissue with dense connective tissue. There was mild inflammation in all groups. The histological assessment determined that wounds treated with 5% TRF had been healed and were comparable to the uninjured skin, whereas the wounds in the other groups were nearing the end of the healing process.Table 5.
|
Table 5: The macroscopic image of wound appearance at 7-day intervals. |
||||
|
Treatment/Day |
1 |
7 |
14 |
21 |
|
Cream base |
|
|
|
|
|
3% TRF |
|
|
|
|
|
4% TRF |
|
|
|
|
|
5% TRF |
|
|
|
|
|
SSD |
|
|
|
|
DISCUSSION
Wound healing in any tissue follows a specific sequence of events [13], initiated with haemostasis, inflammation, proliferation, and finally remodeling phases [14]. These phases must occur within a specific time and continue for a specific duration at an optimal intensity [15]. The optimal process of wound healing is necessary for restoring normal skin conditions within the shortest time possible. There are incomplete repair and scar contracture in wounds in which healing is impaired.
Vitamin E exists in tocotrienol and tocopherol form. Both isomers exhibit a potent lipophilic antioxidant function, preventing lipid peroxidation and resulting in more stable cell membranes [16]. Our main objective was to compare the effect of TRF and SSD treatments on burn wound healing. We found that TRF healed the wounds within a significantly shorter duration. The parameters used in this study were wound contraction rate, clinical evaluation, and histological observation on day 0, 7, 14 and 21 post-burn. There were no signs of macroscopic or microscopic infection in the TRF-treated groups, indicating the antimicrobial properties of TRF. This observation is parallel to the study by Pierpaoli and colleagues, who reported increased antibacterial properties upon vitamin E supplementation in an animal model [17].
The rate of wound contraction is a useful means of assessing the progress of wound healing, as it reflects the number and contractile property of myofibroblasts that will orient themselves along the lines of tension and draw collagen fibers together, resulting in wound contraction. Myofibroblasts may promote lesion retraction of 50%–70% of the original wound [18]. In the present study, 3%, 4%, and 5% TRF increased the rate of the wound contraction, which was in agreement with a previous study that reported an increased rate of wound contraction following oral supplementation of tocopherol [19]. Previous studies have also shown that vitamin E scavenges free radicals and wholly prevents the free radical–induced alteration of collagen and glycosaminoglycan. In addition, vitamin E has a stabilizing effect on the lysosomal membrane, which is important for collagen biosynthesis [20]. Hence, it is possible that TRF increases the rate of wound contraction by increasing collagen synthesis, subsequently stimulating fibroblast transformation into myofibroblasts [21].
The gross appearance and clinical evaluations revealed that the sequential biological events were accelerated in the TRF-treated groups. The macroscopic findings on day 12 postburn were related to the faster granulation tissue formation, re-epithelialization, and collagen deposition. The clinical evaluation determined that TRF reduced hyperemia at a later stage and significantly decreased the re-epithelialization period, crusting period, and scar tissue formation. The complete reepithelialization showed that the mitosis of viable epithelial cells had formed a complete epithelial bridge. Faster reepithelialization indicates faster epithelial cell and keratinocyte proliferation and migration. A recent study showed that TRF stimulated epithelial cell mitosis [22]. In addition, the application of TRF kept the wounds moist; it has been proven that keratinocytes migrate more easily over a moist wound surface than underneath a dry scab [23]. In the 4% TRF–treated group, the complete re-epithelialization at day 14 and the hair follicles lining the wound margins indicated a concentric decrease in the size of the open wound.
Histopathological evaluation of the wound site was also performed to confirm the morphometric findings. The histopathological data of the TRF-treated wounds on day 7, 14, and 21 indicated a more advanced repair process than in both control wounds. The H&E staining revealed that topical application of TRF enhanced re-epithelialization at day 7, stimulated granulation tissue formation at day 14, and stimulated the healing process by regeneration instead of fibrosis at day 21. It was predicted that TRF potentially enhanced reepithelialization by increasing epithelial cell proliferation and migration across the wound surface. This result was supported by the study of Zampieri and colleagues, who stated that topical vitamin E treatment created a moist healing environment that enhanced epithelialization in patients of all ages [24]. At day 7, 14 and 21, the TRF-treated groups had dense connective tissue and abundant fibroblasts as compared to both of the control groups, which is parallel to the study by Rashid and colleagues, who demonstrated that TRF stimulates fibroblast production of basic fibroblast growth factor, which is important for improving quality and quantity of collagen tissue and extracellular matrix [25]. The reduced inflammation in the TRF-treated groups at day 7 and 14 showed that TRF might have anti-inflammatory properties, which aids in reducing inflammatory cell infiltration. This finding is supported by a previous study that proved that vitamin E exhibits anti-inflammatory activity [26,24]. Controlling the rate of inflammation during the wound healing process is very important, as high levels of inflammatory cell infiltrate may lead to an abnormal repair response and might delay wound healing [27]. TRF has potential in scavenging free radicals, thus helps arrest the chain propagation to prevent further damage to the tissue and enhance the wound healing process. Our results provide evidence that TRF stimulates the healing process by reducing the inflammatory response and enhancing reepithelialization.
CONCLUSIONS
TRF heals burns effectively with faster recovery process based on microscopically and macroscopically observation with a shorter duration in each recovery phases compared to SSD. Further studies on the cellular and molecular mechanism of TRF in wound healing are warranted.
ACKNOWLEDGMENTS
This study was supported by a research grant from the Ministry of Science, Technology, and Innovation, Malaysia.








































































































































