Doxorubicin in Combined Therapies: Multifunctional Nanoparticles for Cancer Chemohyperthermia
- 1. Department of Biomedical Engineering (Center of Excellence), Amirkabir University of Technology, Iran
Citation
Yazdanpanah A, Ghaffari M, Moztarzadeh F (2017) Doxorubicin in Combined Therapies: Multifunctional Nanoparticles for Cancer Chemo-hyperthermia. J Drug Des Res 4(1): 1032.
INTRODUCTION
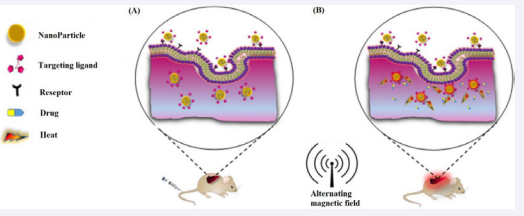
While the combination of hyperthermia and chemotherapy is a reasonable result of its usage with radiotherapy, information around their interactions is far less well studied (Figure 1).
Figure 1: Combination of chemotherapy and hyperthermia. (A) Before applying magnetic field and, (B) After applying magnetic field
Meanwhile exclusive experimental data is now only available in vitro. For the purposes of this application, cytotoxicity of chemotherapeutic agents are catechized into three types [1]. The first group contains those drugs which their cytotoxicity increase linearly with increasing temperature; this group contains alkylating agents, cisplatin and mitomycin-Co. The second group is a class of agents whose cytotoxicity is increase only above a threshold temperature, typically 41 to 42° C. These drugs contain doxorubicin, bleomycin, and actinomycin-D. Third, there are drugs which are not normally associated with cytotoxicity at 37°C, but become cytotoxic at temperature higher than 41 to 42° C. The prototype drug in this category is the anti-fungal agent amphotericin-B. Normally used chemotherapeutic agents which do not show enhanced cytotoxicity with increasing temperature include 5-fluorouracil and the several Vinca alkaloids [2-4].
AFFECTING FACTORS ON CHEMOHYPERTHERMIA
The relations between hyperthermia and chemotherapeutic agents become more complicated by the way in which these agents interact. Insofar as a cytotoxic drug, the drug must be present in the intracellular compartment to act, and membrane permeability will affect drug activity. Hyperthermia is known to show a substantial effect on the cell membrane [5]. For this reason, the sequencing of hyperthermia and different cytotoxic agents might be important in any interaction which might be formed. A group of studies has been reported the complexity of the demonstrating interactions between hyperthermia and chemotherapy as well as designing a rational protocol for their applications [4]. If treatment with hyperthermia enhances cell membrane permeability one would suppose that either simultaneous administration of hyperthermia and doxorubicin or doxorubicin following hyperthermia would prove synergism. Whereas doxorubicin administration preceding hyperthermia would be unlikely to prove such a synergism [6]. The opinion that doxorubicin administration following hyperthermic treatment caused a lower cytotoxic effect from doxorubicin was originally poorly clarified. But, current information on heat shock proteins provides a description for this effect. Cells treated with hyperthermia develop thermal tolerance as an effect of the elucidation of heat-shock proteins which begins during and in the immediate post-treatment period. Heat-shock proteins act in many ways to stabilize the cell membrane response to the hyperthermic test [7,8]. In the instant post hyperthermic period, this increased membrane stability will decrease the probability of a chemotherapeutic agent would enter the cell and produce cytotoxicity. Inactivation of cells by heat is largely a result of membrane inactivation, and there is a very sharp threshold at approximately 42° C. All of the previous observations show an explanation for the mechanism of interaction between hyperthermia and doxorubicin [9,10].
Another factor complicating the interaction of hyperthermia and chemotherapy is the relative structure of a tumor. Tumors have very heterogeneous histology, with areas of necrosis, areas where heavily infiltrated with inflammatory cells, and areas with structures that have the appearance of normal tissues. Perfusion in these several areas is obviously different, and necrotic regions of the tumor will be under perfused and not receive substantial drug concentrations. But, these regions of the tumor cannot easily dissipate heat and easily reach to the higher temperatures [6]. Observing temperatures in these areas is possibly to result in an overestimation of the temperature in well perfused areas of the tumor where a potentially therapeutic concentration of the drug may exist. A consequence to this effect of dissimilarities in perfusion is that at high temperature, vascular collapse resulting in under perfusion and following by overheating and under-concentration of drug may occur. It has been proved that vascular collapse will certainly occur at temperatures above 44°C. Thus, the activation threshold of a certain class of drugs at 42° C leads a too small therapeutic window for the normal use of this combined modality [9]. Drugs which prove a linear increase in their biological effects with heat are easier to use in rational treatment protocols. Any increase in temperature while the drug is present will exhibit an enhanced result in the heated area. There is no need to guarantee reaching a threshold temperature and at the same time not exceed a temperature which will certainly result in vascular collapse in the heated area. Drugs in this category include the simple alkylates as well as the bi-functional alkylates along with some agents having additional properties such as mitomycin-C and cisplatin [11]. No studies have proved a time dependency for the administration of hyperthermia and these drugs. There is some suggestions that at least for Cytoxan, which must be changed by microsomal metabolism to an active alkylating moiety, this change is improved at higher temperatures within the cells of the tumor. Yet, the only known source of the active metabolite of patients is the liver and this is not commonly in the hyperthermia field except when it is the subject of treatment [12]. Alternatively, simple first order thermodynamic effects on chemical kinetics would be enough to clarify the experimental results with this class of drugs, and studies need to be performed to prove this proposal. The group of drugs which becomes cytotoxic at higher temperature maybe offers the most potential for studies. Simple ethyl alcohol falls in this category, as do the caine anesthetics, nevertheless, the best studied drug is amphotericin-B. Many other drugs which have not been studied yet are potential applicants for this class of materials and could offer an extremely powerful therapeutic effects [13]. Drugs free of dose-limiting toxicity at rational concentrations which become cytotoxic only in the heated volume would allow extended and repeated treatments without concern for normal tissue tolerance. Unfortunately, amphotericin-B is quite toxic in its own right and cannot be used with impunity [14]. Lidocaine has acute cardiac toxicity when administered systemically and this issue limits its applications. Both of these agents are membrane active drugs and it is likely that this bears on the mechanism of action which results in cytotoxicity in the presence of hyperthermia.
CONCLUSION
The aim of studies, investigating other possible membrane active drugs, could result in discovery of a suitable combination with therapeutic efficiency within the heated volume. In vivo studies of combined hyperthermia and chemotherapy have been limited to animal models. The in vitro release kinetics can vary from a few minutes to often quite long, 48 h or more. This must be well-matched with an in vivo application, allowing for the time for the nanocarriers to reach the targeted site and enter cells. In a clinical study, Lübbe et al. [15], applied a magnetic field for 60-120 min in order to accumulate their SPIONs in a tumor. An ideal release profile would have only a moderate release during the time of magnetic focalization, to avoid distributing a high percentage of doxorubicin in the organism. This delay in doxorubicin release must also be considered for theranostic nanosystems (doxorubicin release and imaging). The residence time of nanocarriers in cells must be explored, to evaluate the interest of a long sustained release. Clinical studies today have been limited to continuing test studies primarily using alkylating agents or cisplatin. The lack of a threshold temperature and the need for suitable timing of the modalities have made these drugs the most likely ones to produce successful clinical results in initial studies.