Effects of Cyclodextrin Glycosiltranferase Modified Starch and Alfa and Beta Cyclodextrins on Plasma Glucose and Lipids Metabolism in Mice
- 1. Institute of Agrochemistry and Food Technology (IATA-CSIC), Avenida Agustin Escardino, Spain
- 2. S. Department of Agriculture, Processed Foods Research, Agricultural Research Service, USA
Abstract
The potential functional and nutritional benefits of granular starch treated with cyclodextrin glycosyltransferase (CGTase) and the released cyclodextrins (CDs) were explored in in vivo studies. The metabolic effects of diets in the C57BL/6J mouse containing native and enzymatically modified corn starch by CGTase with or without hydrolysis products were studied. The hydrolysis products were oligosaccharides and CDs, mainly b-CD. Blood glucose concentration at 2 hours was higher suggesting that enzymatically treated starches containing CDs slowed digestion resulting in a longer period of absorption and consequently higher blood glucose levels at the later times. The modified starch with CDs tended to increase HDL-cholesterol levels while decreasing VLDL-cholesterol levels. The CGTase modified starches lowered total and cholesterol ester values in liver and decreased fecal fat extraction. The inclusion of CGTase modified granular starches in the presence of their hydrolysis products may be useful to prevent obesity and other related metabolic diseases, offering an alternative healthy ingredient to the food industry.
Keywords
• Starch
• Cyclodextrin
• Metabolic effect
• Lipids
Citation
Dura A, Yokoyama W, Rosell CM (2017) Effects of Cyclodextrin Glycosiltranferase Modified Starch and Alfa and Beta Cyclodextrins on Plasma Glucose and Lipids Metabolism in Mice. J Drug Des Res 4(5): 1051.
ABBREVIATIONS
CGTase: Cyclodextrin glycosyltransferase; CDs: cyclodextrins; HDL: High Density Lipoprotein; VDL: Very Low Density Lipoprotein; LDL: Low Density Lipoprotein; CGT-W: Washed Enzymatically Treated Corn Starch; CGT-NW: Enzymatically Treated Corn Starch Containing the Hydrolysis Products; GTT: Glucose Tolerance Test.
INTRODUCTION
Granular starches can be effectively modified by enzymes leading to porous structures with higher adsorption ability [1]. In fact, they have been used as carriers for providing protection of minerals, vitamins, flavors and lipids [2]. Enzymatically modified starches have stimulated interest owing to their potential to modulate starch digestion, glycemic response, and plasma and liver lipids by interactions with fatty acids and cholesterol and/or bile acids in the intestinal lumen. Most in vivo and in vitro studies have focused on starch digestibility, glycemic response and metabolic effects based on a starch’s glycemic index (GI), relating slow digestible and resistant starch in carbohydrate matrices to low GI foods. Furthermore, enzymatically modified starches has been studied for their possible healthy effect. Particularly, they have been proposed to increase resistant starch content when treated with pullulanase [3], to increase the amount of slowly digested starch when using β-amylase, transglucosidase and maltogenic α-amylase treatment [4] or to reduce postpandrial glycemic response in rats by α-amylase treatment in corn starch [5].
Cyclodextrin glycosyltransferase (CGTase) is an endoenzyme that catalyzes three transglycosylation reactions: cyclization, coupling, and disproportionation by cleaving interior α-1,4 glycosidic bonds of starch molecules. The major activity is cyclization leading to the formation of non-reducing cyclic oligosaccharides or cyclodextrins (CDs) [6]. The main types of CDs are α-, β-, and γ-CDs consisting of six, seven, and eight glucose monomers in a cyclic configuration, respectively [7]. CDs are arranged with an outer hydrophilic surface that makes them soluble in water and a hydrophobic cavity that facilitates the formation of complexes with a wide variety of hydrophobic guest molecules. Many water insoluble organic molecules have been incorporated and solubilized by complex formation and found uses in pharmaceutical, food, cosmetics, analytical chemistry, agriculture, and biotechnology [8]. CDs may form complexes with fatty acids and emulsifiers, in fact, β-cyclodextrin has been reported to reduce cholesterol availability in a wide variety of foods [9].
CGTase is also a useful enzyme for modifying the structure of granular starch leading to surface pores, which number and size can be modulated by controlling the level of enzyme [1]. Resulting porous starches showed altered technological properties like pasting and thermal behavior. Furthermore, those starch properties could be changed when the released hydrolysis products are kept together to the starch structure [10]. Nevertheless, enzymatic modification by CGTase and its relation to starch digestion, absorption and metabolic effects have never been attempted, despite the complexing ability of the cyclodextrins. Therefore, the aim of this study was to investigate and compare the metabolic effects of high fat diets in the C57BL/6J mouse containing native and enzymatically modified corn starch by CGTase and also the possible contribution of the cyclodextrin released from the enzymatic action.
MATERIALS AND METHODS
Corn starch samples were generously supplied by HuiciLeidan (Navarra, Spain). Cyclodextrin glycosyltransferase (CGTase, EC 2.4.1.19, (Toruzyme® 3.0 L, declared activity 3KNU/mL product) of food grade was provided by Novozymes (Bagsværd, Denmark). Analytical grade reagents were purchased from Sigma-Aldrich (Madrid, Spain).
Sample preparation
The CGTase modification of corn starch has been previously described by Dura et al. [11]. Briefly, corn starch (10.0 g) was suspended in 50 mL of 20 mM sodiumphosphate buffer at pH 6.0. The enzyme treated starches were prepared by adding CGTase (0.32 U of CGTase /10 g starch) to the starch suspension. The suspension was placed in a shaking water bath at 50º C for 48h. 50 mL of water was added to the suspension and homogenized with a PolytronUltraturrax homogenizer IKA-T18 (IKA works, Wilmington, DE, USA) for 1 min at speed 3. Samples were centrifuged for 15 min at 7,000×g at 4º C. The starch pellets were washed with 50 mL of water and centrifuged again using the conditions described above. Supernatants were pooled and boiled in a water bath for 10 min to inactivate the enzyme. Two enzymatically treated corn starches were prepared. One sample was the washed enzymatically treated corn starch (CGT-W) and in the other the hydrolysis products were added back (CGT-NW) to assess the role of the water soluble hydrolysis products. Both samples were freeze-dried and kept at 4º C for further analyses. Native corn starch (N) without treatment was used as the control sample.
CDS and oligosaccharides quantification high performance anion exchange chromatography
Oligosaccharides and CDs in freeze-dried hydrolysates were analyzed by HPAEC using a CarboPac PA-100 column (250 mm × 4 mm) for separation and coupled to a pulsed amperometric detector (Dionex, Sunnyvale, CA). The flow rate was 1.0 mL/min and the injection volume was 10 μL. A ternary gradient was used: A (water), B (1 M NaOH), C (1 M C2 H3 NaO2 ), and D (water), the following running profile was applied: time zero, 46.25% A, 5% B, 2.5% C, 46.25% D; 25 min, 42.5% A, 5% B, 10% C, 42.5% D; 1 min, 35% A, 15% B, 15% C, 35% D; 3 min, 33% A, 15% B, 19% C, 33% D; 5 min, 28.5% A, 15% B, 28% C, 28.5% D; 1.5 min, 18.5% A, 15% B, 48% C, 18.5% D. Standards were used to identify and quantitate each compound. Samples were analyzed in duplicate.
Mice and diets
Twenty four male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Me, USA). The mice were housed individually in an environmentally controlled room (20- 22º C, 60% relative humidity, 12 h alternating light: dark cycle). Mice were acclimatized and given ad libitum access to water and mouse chow diet (LabDiet, PMI International, Redwood, CA, USA; protein, 239 g/kg; fat, 50 g/kg; non-nitrogenous substances, 487 g/kg; crude fiber, 51 g/kg; ash, 70 g/kg; energy, 17 MJ/kg; and sufficient amounts of minerals and vitamins for healthy maintenance) for one week prior to the initiation of the experimental diets. Mice were weighed and randomized into three groups of eight mice and fed for two months. The control group was fed native corn starch (N) and the other two groups of mice were fed either CGT-W or CGT-NW enzymatically modified samples (Table 1).
Table 1: Composition of experimental diets.
| Diet type | |||
| Ingredient | N | CGT-W | CGT-NW |
| Lard Fat | 22 | 22 | 22 |
| Soybean Oil | 25 | 25 | 25 |
| Cholesterol | 0.8 | 0.8 | 0.8 |
| MCC* | 50 | 50 | 50 |
| CGT-W corn starch | 0 | 400 | 0 |
| CGT-NW corn starch | 0 | 0 | 400 |
| N Corn Starch | 400 | 0 | 0 |
| Casein | 200 | 200 | 200 |
| Sucrose | 48.2 | 48.2 | 48.2 |
| L-Cystine | 3 | 3 | 3 |
| Choline Bitartrate | 3 | 3 | 3 |
| Mineral Mix | 35 | 35 | 35 |
| Vitamin Mix | 10 | 10 | 10 |
| Total Diet (g) | 1000 | 1000 | 1000 |
| Abbreviations: MCC (microcrystalline cellulose, control): non-viscous water insoluble fiber; N = Native corn starch diet; CGT-W = washed corn starch diet treated with CGTase; CGT-NW = non-washed corn starch diet treated with CGTase |
|||
Mice were fed ad libitum with high fat (HF) diets containing 17% of energy as protein, 37% as carbohydrate, 47% as fat with 0.1% cholesterol and 5% microcrystalline cellulose (MCC; Dyets Inc. Bethlehem, PA).
Body weights were recorded weekly and food intake was monitored twice per week. The study protocol, #P- 09–04, was approved by the Animal Care and Use Committee, Western Regional Research Center, USDA, Albany, CA, USA.
Metabolic effect of the diet
The glucose tolerance test (GTT) was administered 1 week before the end of the feeding study. The mice were fasted for 3 h before administration of glucose (2 g/kg body weight) by gavage. Capillary blood was taken from the tail vein at 0, 15, 30, 60, and 120 min and glucose was determined using a One Touch Ultrameter (LifeScan Inc., Fremont, CA, USA).
For the plasma and adipose tissue collection, mice were feed deprived for 12 h and anesthetized with Isoflurane (Phoenix Pharmaceutical, St. Joseph, MO, USA). Blood was collected by cardiac puncture with syringes previously rinsed with potassium EDTA solution (15% w/v). The plasma was separated after centrifugation at 2000 × g for 30 min at 4º C. Epididymal and subcutaneous adipose tissues were collected, weighed, and immediately frozen in liquid nitrogen for analysis.
Plasma lipoprotein cholesterol was determined by separation of the lipoprotein particles by HPLC size-exclusion chromatography with post-column colorimetric detection by enzymatic cholesterol oxidase reagent as previously described by German et al. [12].
Fat was extracted from the liver with hexane: isopropanol (3:2) using a high pressure and temperature automated extractor (ASE 200, Dionex Corp., Sunnyvale, CA) as described previously by Hong et al. [13]. Hepatic triglycerides, total cholesterol, and free cholesterol were determined by enzymatic colorimetric assays using commercial kits (Genzyme Diagnostics PEI Inc., PE, Canada, Roche Diagnostics, Indianapolis, IN, and Wako Chemicals, Richmond, VA).
Total lipid content was determined in the feces, which were collected during the last 3 consecutive days of the feeding period and were stored at -80º C. Fecal total lipid contents were determined gravimetrically after solvent extraction (ASE 200, Dionex Corp., Sunnyvale, CA) as described elsewhere [13].
STATISTICAL ANALYSIS
Experimental data were subjected to analysis of variance (ANOVA) using Statgraphics Centurion XV software (Bitstream, Cambridge, N). When analysis of variance indicated significant F values, multiple sample comparisons were also performed by Fisher’s least significant differences (LSD) test to differentiate means with 95% confidence.
RESULTS AND DISCUSSION
CDs and oligosaccharides quantification by High Performance Anion Exchange Chromatography
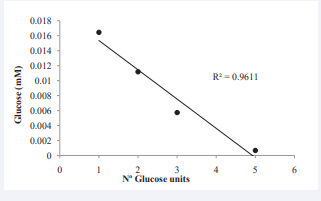
Oligosaccharide and CD composition of the CGTase treated starches were analyzed to confirm the existence of hydrolysis products in the treated starches. As expected, there were no detectable oligosaccharides or CDs neither in native starch and CGT-W, confirming the removal of the hydrolysis products after washing. In the case of CGT-NW sample, hydrolysis products were kept and the presence of oligosaccharides as glucose, maltose, maltotriose, maltotetraose andmaltopentaose was detected (Figure 1).
Figure 1: Contents of glucose, maltose, maltotriose, maltopentaose, α-cyclodextrin and β-cyclodextrin in mg 100 g-1 of starch in CGT-NW samples.
It was observed that the molar ratio of glucose, maltose, maltotriose, maltotetraose, and maltopentaose linearly decreased with increasing molecular weight. Regarding cyclodextrins production, although the most common CDs are α-, β-, and γ-CD [14], only α-CD (3.88 mg/100 g starch) and β-CD (6.04 mg/100 g starch) were extracted and quantified. Results confirmed that CGTases, in addition to catalyze transglycosylation and cyclization reactions, produce oligosaccharides with different degrees of polymerization through hydrolysis or disproportionation [6].
Effect of CGTase modified starch and CDs on body weight, plasma and adipose tissue collection
Final body weights and weight gains were not different between mice on the native corn starch diet and enzymatically modified corn starch diets (Table 2).
Table 2: Effect of experimental diets on Body Weight, Body Weight Gain, Food Intake, Feed efficiency, Liver, Kidney and Adipose Tissue Weigh .
| N | CGT-W | CGT-NW | |
| Body weight (g) | 31.2±4.9 | 31.9±3.8 | 32.8±4.0 |
| Body weight gain (g) | 29.8±4.3 | 30.4±3.3 | 31.4±3.3 |
| Food intake (g/day) | 133.9±6.2a | 110.0±7.9b | 108.9±8.3b |
| Feed efficiency | 4.8±0.5a | 3.7±0.4b | 3.5±0.4b |
| Liver weight (g) | 0.9±0.1 | 0.9±0.1 | 0.9±0.1 |
| Kidney weight (g) | 0.2±0.0 | 0.2±0.0 | 0.2±0.0 |
| EA (g) | 1.4±0.5 | 1.5±0.6 | 1.3±0.8 |
| a Data presented as means ± SE. Different letters indicate significant difference at P < 0.05. EA = epididymal adipose; N = Native corn starch diet; CGT-W = washed corn starch diet treated with CGTase; CGT-NW = non-washed corn starch diet treated with CGTase |
|||
Mice on the native corn starch diet had higher feed efficiency (g feed/g body weight gain) and food intake (P < 0.05), despite similar body weight and weight gain to the CGTase treated starch groups.
for identification of potentially harmful effects. There was no difference in kidney weight due to diet changes, which was expected since the kidney does not participate in fat or carbohydrate metabolism, and the similar weights were indicative of healthy animals. No differences in liver weight among all groups were observed, following the pattern of the body weight, indicating an adequate physiological response of the organism [15]. Therefore, considering that all diets were high fat, differences among type of starch in the diet did not affect liver weight and subsequent weight gain, which only seems to be affected when comparing diets with different levels of fat [16].
Only the weight of epididymal adipose from mice fed CGT-W tended to be higher when compared to the other two diets (Table 2).
Effect of CGTase treated starch and CDs on glucose tolerance test (GTT)
The impact of GTase treated starch and cyclodextrins intake on the glucose tolerance test was tested. Marked differences in blood glucose levels between the native corn starch control diet and the CGTase treated starches after oral gavage of glucose were detected (Figure 2).
Figure 2: Glucose tolerance in mice gavage with glucose (2 g/kg body weight) after fasting for 3 hours and area under the curve (AUC) values. The initial blood glucose values were adjusted to 0 and the subsequent values adjusted by subtracting the initial value. The samples studied were native corn starch diet (?), washed corn starch diet treated with CGTase (♦) and non-washed corn starch diet treated with CGTase (?). Data are expressed as mean n = 8/group. Error bars represents the standard deviation.
The time to reach the maximum glucose peak was about the same (20 min), but beyond that plots showed different trends. In mice on the native corn starch diet, the glucose concentration peaked sharply and then returned almost to baseline at 2 hrs. In contrast, the blood glucose levels of mice fed the CGT-NW treated starch remained higher longer, suggesting the mice have become insulin resistant on the high fat diet or the starch was less digestible. The glucose plot observed when feeding CGT-W displayed lower glucose concentration than those obtained with the other diets during the first hour, but showed steady levels longer. Differences observed between CGT-W and CGT-NW diets might be ascribed to the presence of free sugars and oligosaccharides in the CGT-NW. Moreover, the glucose pattern obtained with the CGTase treated starches supported the assumption that enzymatically modified starches were less digestible. It has been previously reported that the administration of resistant starch reduced blood glucose level due to the promotion of glycogen synthesis and inhibition of gluconeogenesis [17]. After enzymatic modification with CGTase at sub-gelatinization temperature, a number of possible changes in starch granule structure and biochemical properties may occur that affects glucose disposal to its absorption into the blood. In fact, previous studies reported that enzymatic treatment of the starch by CGTase seems to affect the amorphous zone, releasing sugars from those accessible chains, and leading to a more crystalline structure that was more resistant to the amylase hydrolysis [10,18].
The area under the curve (AUC) for native and CGT-W samples was similar and lower than CGT-NW sample. The rate of carbohydrate digestion has been indexed by comparing the AUC of a carbohydrate source to white bread containing the same amount of starch, usually 50 g available carbohydrate. The glycemic index (GI) has been related to metabolic diseases and studies have reported the benefits of low GI diets to improve metabolic syndrome [19]. The tendency to higher epididymal adipose weights from mice fed CGT-W is in contrast to most studies that intake of slower digestible carbohydrates (low GI) decrease adiposity compared to high GI diet in Wistar rats [20], diabetic Sprague-Dawley rats, C57BL/6J mice [21] and in 129SvPas mice [22,23], thus the enzymatic modification of corn starch promoted by CGTase resulted in slower digestible carbohydrates that induced different metabolism.
Impact of CGTase treated starch and CDs on lipid metabolism
To determine the impact of CGTase treated starches and cyclodextrins on the lipid metabolism, the plasma cholesterol distribution and the analysis of lipids in liver and feces were determined.
Table 3: Plasma cholesterol distributio.
| N | CGT-W | CGT-NW | |
| VLDL (mg/dL) | 561±87b** | 562±104b** | 381±140a** |
| LDL (mg/dL) | 1945±750a* | 2985±793b* | 2387±858ab* |
| HDL (mg/dL) | 15755±1703 | 15972±1799 | 16887±919 |
| TC (mg/dL) | 18012±1995 | 19595±1852 | 19258±1468 |
| LDL/HLD | 0.125±0.037a* | 0.182±0.039b* | 0.145±0.052ab* |
| **Values are means ± SD. Means with the same letter in a roware not significantly different from each other (P<0.05).* Values are means ± SD. Means with the same letter in a row are not significantly different from each other (P<0.1).N = Native corn starch diet; CGT-W = washed corn starch diet treated with CGTase; CGT-NW = non-washed corn starch diet treated with CGTase; TC = total cholesterol |
|||
Table 4: Effects of experimental diets on hepatic lipid and cholesterol concentrations in mic .
| N | CGT-W | CGT-NW | |
| Total lipid (%) | 8.0±0.3 | 8.2±0.8 | 7.0±0.5 |
| TC (μg/g liver) | 101.5±29.7b | 94.7±16.6b | 58.4±17.3a |
| FC (μg/g liver) | 11.4±4.5 | 12.8±4.3 | 10.2±4.7 |
| EC (μg/g liver) | 90.1±17.8b | 81.9±8.7b | 48.2±8.9a |
| Ester Ratio (%) | 88.8 | 86.5 | 82.5 |
| a Data presented as means ± SE. Different letters within a row indicate significant difference at P < 0.05. TC = total cholesterol; FC = free cholesterol; EC = Ester cholesterol; N = Native corn starch diet; CGT-W = washed corn starch diet treated with CGTase; CGT-NW = non-washed corn starch diet treated with CGTase |
|||
Plasma lipoprotein cholesterol results are presented in (Table 3). No significant difference was found regarding total cholesterol content owing to these particular diets, but significant differences were observed concerning the plasma cholesterol distribution. Significant higher levels of LDL cholesterol in plasma were quantified in mice fed the diet CGT-W diet (P < 0.01), likely due to the absorption ability of the porous starch resulting from the enzymatic treatment [24]. Consumption of CGT-NW diets tended to increase the level of HDL cholesterol when compared with N and CGT-W diets. Moreover, the level of VLDL cholesterol was significantly lower (P < 0.05) for mice fed the CGT-NW diet compared with the other diets. The CGT-NW also tended to lower LDL cholesterol and a tendency to lower LDL/HDL ratio being significantly different (P < 0.01) from enzymatically modified sample without hydrolysis products, CGT-W. β-CDs is a cholesterol sequestrant that effectively removes cholesterol from animal products improving their nutritional characteristics [8,25]. There is a possibility that cholesterol in the intestinal lumen may be bound by β-CDs formed by the CGTase hydrolysis of starch and excreted in the feces. In order to maintain homeostasis, fecal cholesterol loss results in increased uptake of LDL cholesterol from the blood thus reducing blood cholesterol levels. Considering the adsorption ability of the porous starch and the cyclodextrins capacity to form inclusion complexes with hydrophobic compounds, it seems that only the inclusion of CGTase treated starches containing cyclodextrins would allow reducing the plasma cholesterol counteracting the absorption ability of the porous starch.
In addition to insulin resistance and high fasting blood glucose, fatty liver, increased plasma lipids and high blood pressure are characteristics of metabolic disease. Liver lipid profile is presented in (Table 4). Generally, the CGTase treated corn starch diets tended to reduce total and esterified cholesterol concentrations. However, levels of fat in the liver were clearly lower from mice fed the CGT-NW corn starch diet, which supported the observed differences in plasma cholesterol in mice fed the CGT-NW diet. In particular, total cholesterol and ester cholesterol were significantly lower in the CGT-NW group compared with the mice fed with the other two diets (P < 0.05). High fat diet may induce hepatic steatosis and signs of hepatic insulin resistance in the animals, as also observed in obese humans [16]. Present results confirmed that a high fat diet having enzymatically modified corn starch containing cyclodextrins (CGT-NW)led to improve lipid liver profile in mice, as well as plasma lipoprotein cholesterol.
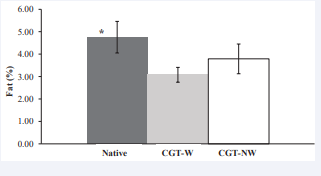
Figure 3: Total lipid extraction in feces in male mice fed with Native corn starch (N), washed corn starch treated with CGTase (CGT-W) or non-washed corn starch treated with CGTase (CGT-NW). * Denotes significant difference at P < 0.05.Error bars represents the standard deviation.
When lipid content in feces was determined (Figure 3), total fecal lipid excretion was significantly higher (P < 0.05) in mice treated with native corn starch diet compared to mice fed with enzymatically treated corn starch diets. This result would explain differences in food intake and feed efficiency previously mentioned. Mice on the native starch diet had higher food intake and feed efficiency than mice on the CGTase treated starch due to higher fat extraction in feces that resulted in maintenance of body weight values. Again, the adsorption capacity of the porous starches could be responsible of the lower excretion of the lipids.
CONCLUSION
Determining the metabolic effect of CGTase modified granular starch with or without hydrolysis products (oligosaccharides and cyclodextrins) in the C57BL/6J mouse subjected to a high fat diet, provided a new perspective in order to modify food properties aiming at improving their functionality and their impact in human health. The presence/absence of hydrolysis products in CGTase modified starches headed to metabolic differences among mice in relation to glycemic response and lipid metabolism. CGTase treated starch induced a decrease in food intake, longer steady levels of blood glucose, an increase in the levels of plasma cholesterol, and reduced levels of lipids in liver and feces. Besides those effects, the presence of cyclodextrins in CGT-NW diet conducted to insulin resistant mice response and lower cholesterol content, increasing HDL-cholesterol values while decreasing VLDL-cholesterol. Overall, CGTase modification of granular starch offers an additional possibility to obtain healthier food ingredients.
ACKNOWLEDGEMENTS
Authors acknowledge the financial support of the Spanish Ministry of Economy and Competitiveness (Project AGL2011- 23802 and AGL2014-52928-C2-1-R), the European Regional Development Fund (FEDER. A. Dura would like to thank predoctoral fellowship from Spanish Ministry of Economy and Competitiveness. This research was performed in part at the Processed Foods Research, Agricultural Research Service, U.S. Department of Agriculture, Albany, California 94710, United States.