Factors Influencing the Proliferation and Differentiation of Inner Ear Stem Cells
- 1. Department of Otorhinolaryngology, the Third Hospital of Jilin University, China
Abstract
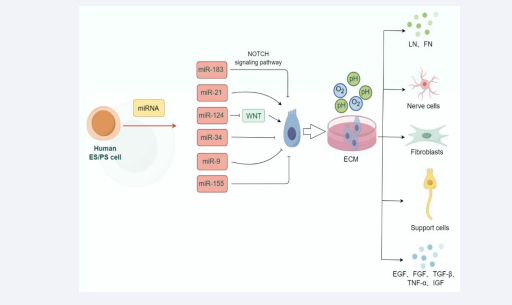
Irreversible damage to cochlear hair cells is one of the main causes of sensorineural deafness. In recent years, it has been found that a class of cells with stem cell properties and the ability to differentiate into hair cells, which are called inner ear stem cells, and their proliferation and differentiation may be the ultimate solution of sensorineural deafness. Inner ear stem cells have the potential for self- renewal and multi-differentiation, and their proliferation and differentiation processes are affected by a variety of factors, ranging from growth factors and miRNAs to the extracellular microenvironment and related signaling pathways, etc. This paper focuses on the factors affecting the proliferation and differentiation of inner ear stem cells.
KEYWORDS
- Inner Ear Stem Cells
- Regeneration
- Signaling Pathway
CITATION
Zhu X, Liu Y, Hao Y (2024) Factors Influencing the Proliferation and Differentiation of Inner Ear Stem Cells. J Ear Nose Throat Disord 8(1): 1057.
INTRODUCTION
Inner ear stem cells were initially isolated from the sensory epithelium of the elliptic bursa of adult mice by Li et al., in 2003 and were named inner ear stem cells [1]. Since then researchers have isolated such cells from the cochlear Corti apparatus, the large epithelial ridge, and the small epithelial ridge [2,3]. Inner ear stem cells have the potential for self-renewal and multidirectional differentiation, which have a greater potential to differentiate into cochlear hair cells than other types of transplanted stem cells. Cochlear hair cells are terminally differentiated cells, whose irreversible damage or absence is one of the main causes of sensorineural deafness. Many studies have attempted to fundamentally treat sensorineural deafness by culturing inner ear stem cells in vitro , inducing them to differentiate into hair cells or hair cell-like cells. A challenge with this approach is how to regulate cell proliferation and differentiation once the cells have been reprogrammed and undergone differentiation. The differentiation of inner ear stem cells into mature cochlear hair cells is closely related to the microenvironment in which they reside, as well as the exchange of material, signaling, and genetic regulatory.
MicroRNAs
During the differentiation of inner ear stem cells, cells begin to differentiate directionally when specific transcriptional programs of certain genes are activated. MicroRNAs (miRNAs) are a class of small non-coding RNAs processed from the transcripts of endogenous genes, which are involved in a wide range of physiological and pathological processes by regulating the expression of target messenger RNAs (mRNAs). To date, hundreds of miRNAs have been identified in cochlear progenitor cells in culture.
The miR-183 family (including miR-183, miR-96, miR-182) is thought to be essential in the development of the inner ear. They originate from a common primary transcript, and the initial pattern of expression is widely distributed, but as the embryo develops, miR-183 expression is progressively confined to sensory cells in the cochlea and vestibular end-organs, as well as in the spiral ganglion and vestibular ganglia, with the most abundant expression in hair cells [4]. It was found that overexpression of miR-183 in zebrafish induced additional and ectopic hair cells, while low expression reduced the number of hair cells [5]. MiR-183 regulates the behavior of inner ear stem cells by affecting different target genes and signaling pathways, it can target genes that affect the cell cycle to promote or inhibit the proliferation of inner ear stem cells [6,7]. In addition, miR-183 can affect the differentiation of inner ear stem cells to specific cell types such as hair cells by regulating differentiation related signaling pathways such as the Notch signaling pathway [8]. By establishing a gentamicin induced cochlear injury model in mice, Zhou W et al. found that gentamicin-induced hair cell injury activated the Notch signaling pathway and downregulated the expression of miR-183. Inhibition of this signaling upregulated miR-183 cluster expression and promoted hair cell regeneration, suggesting that miR-183 cluster may be involved in notch inhibition-induced hair cell regeneration in gentamicin-treated cochlea.
In recent years, the role of miR-21 in stem cell proliferation and differentiation has received increasing attention, and the regulation of miR-21 significantly affects the proliferative and differentiation capacity of these cells [9,10]. It has been shown that overexpression of miR-21 promotes the proliferation and differentiation of inner ear stem cells to hair cell-like cells, which is important for hair cell regeneration [11]. MiR-21 can also regulate the behavior of inner ear stem cells by targeting several signaling pathways. Among them, PTEN (phosphatidylinositol-3- kinase inhibitor 1) and Spry1 (spray delay protein 1) are known targets of miR-21, and by inhibiting the expression of these target genes, miR-21 can activate the AKT signaling pathway and ERK signaling pathway, which can promote the proliferation and differentiation of cells [12].
Similarly, miR-124 can affect the behavior of inner ear stem cells by binding to the mRNAs of target genes, which may be involved in cell cycle control, cell death, signaling pathways, and other important biological processes, by blocking their translation process or promoting their degradation. It has been shown that miR-124-3p negatively regulates the EYA1 gene by interacting with the 3 ‘ UTR target site of the EYA1 gene, which leads to inner ear dysplasia in zebrafish [13]. Jiang D et al., found that miR-124 was lowly expressed in undifferentiated inner ear neural stem cells, with a gradual increase in expression and a peak at day 14 of differentiation. miR-124 overexpression increased the percentage of neurons and axon length, suggesting that miR-124 plays an important role in neuronal differentiation of inner ear stem cells in vitro [14].
MiR-34 is an important downstream effector of the p53 signaling pathway. P53 participates in cellular response programs, including cell cycle arrest, apoptosis, and aging, by activating miR-34 expression. In inner ear stem cells, p53-activated miR- 34 expression may have an inhibitory effect on cell proliferation and differentiation [15]. The let-7 family is a group of highly conserved microRNAs that were first identified in Caenorhabditis elegans and subsequently characterized in a variety of organisms, including humans. Let-7 family members play important roles in different biological processes, especially in cell proliferation, differentiation, development, and the onset and progression of diseases. They play important roles in various biological processes, especially in cell proliferation, differentiation, development, and the onset and progression of disease. Let-7 is able to directly target and regulate key molecules that affect the cell cycle, such as Cyclin D and Cyclin E, as well as other proteins that promote the entry of cells into the proliferative state. By down-regulating the expression of these proteins, let-7 helps to maintain cells in a quiescent state or promote their exit from the proliferative cycle, which in turn affects the proliferative capacity of inner ear stem cells [16]. Let-7 promotes cell differentiation by targeting multiple transcription factors and signaling molecules associated with the undifferentiated state of cells, such as Hmga2, Myc and Lin28, and inhibiting their expression. In inner ear stem cells, this role of let-7 may help to drive the differentiation of stem cells to specific inner ear cell lines such as hair cells, which is critical for restoring or maintaining hearing function [17]. Let-7 is also involved in the regulation of multiple signaling pathways that are closely linked to cell fate decisions, including Wnt, Notch and TGF-β. By precisely regulating the expression of key molecules in these pathways, let-7 influences the function of inner ear stem cells [18] (Figure 1).
Figure 1: Factors influencing the proliferation and differentiation of inner ear stem cells. Growth factors, miRNA and extracellular microenvironment may affect the viability and proliferation of auditory stem cells.
In addition, miR-9 [19], and miR-155 [20], can also target a series of key genes and pathways to regulate the proliferation, differentiation, and regeneration of inner ear stem cells. With the continuous progress of RNA detection technology, it is expected that more mRNAs will be found in the inner ear in the future, but so far, the exact mechanism by which miRNAs affect inner ear development is still not well understood. Therefore, an in- depth understanding of the specific mechanisms of action of these miRNAs in the proliferation and differentiation of inner ear stem cells will contribute to the development of new therapeutic strategies for hearing loss.
Atoh1 (Atonal homolog 1) is a transcription factor, a member of the bHLH (basic helix-loop-helix) family of proteins, which was first identified in Drosophila and subsequently in mammals, where its homologs are particularly critical for the development of the nervous system [21]. As a transcription factor, Atoh1 activates or represses the expression of a range of downstream genes by binding to specific sequences on DNA. These genes are involved in the regulation of cell growth, differentiation, and the determination of specific cell fates [22,26]. Currently, it has been found that transfection of small activating RNA targeting Atoh1 into inner ear progenitor cells induces differentiation of hair cell progenitors to hair cell-like cells [23].Thus, RNA activation technology has the potential to provide a new strategy for hair cell regeneration. Mcgovern MM et al., reprogrammed non- sensory cells in the vicinity of the organ of Corti with three hair cell transcription factors, Gfi1, Atoh1, and Pou4f3, and found that the non-sensory region of the cochlea produced a large number of hair-cell-like cells and that cells in the vicinity of the reprogrammed hair cells expressed markers for support cells, suggesting that transcription of non-sensory cochlear cells in adult animals factor reprogramming can generate chimeras like the sensory cells in the organ of Corti [24], this approach, if successful, will certainly provide a potential new strategy for treating certain types of hearing loss. Li X et al., converted non-sensory supporting cells from inner ear hair cell-impaired mice into inner hair cells by transiently expressing Atoh1 and permanently expressing Tbx2. The new inner hair cells had similar transcriptomic and electrophysiological properties as wild-type inner hair cells, and the formation efficiency and maturation were higher than those of previous studies. However, there was no significant improvement in hearing in mice with damaged inner hair cells, which may be related to the defective electromechanical transduction function of the newborn inner hair cells [25].
Classical signaling pathways
Wnt signaling pathway is an important cell signaling pathway involved in the regulation of a variety of biological processes, including embryonic development, tissue regeneration, cell proliferation and differentiation, etc. It mainly includes Wnt/β- catenin, Wnt/PCP and Wnt/calcium signaling pathways. In the inner ear, the Wnt signaling pathway also plays a critical role in influencing the proliferation and differentiation of inner ear stem cells. During early inner ear development in mammals, Wnt/β-catenin is involved in the differentiation of the inner ear auditory vesicle and auditory substrate [26], in addition, upregulation of Wnt signaling in cochlear sensory precursors and supporting cells also promotes hair cell differentiation [27]. Chai et al., found that activation of the Wnt/β-catenin signaling pathway promotes proliferation of Lgr5-positive stem cells [28,29], however, only a few of these proliferating stem cells transform into hair cells [30]. It suggests that activation of the Wnt pathway only promotes the regeneration of inner ear stem cells but fails to produce large numbers of new hair cells.
Notch signaling is a highly conserved signaling pathway whose activation depends on direct contact between neighboring cells and mechanical pulling of Notch receptors by Notch ligands [31]. Activation of Notch signaling inhibits the proliferation of inner ear precursor cells, maintaining these cells in an undifferentiated state. This inhibition is achieved by directly suppressing the expression of key regulators of cell cycle progression [32]. Cell division is inhibited, for example, by decreasing the activity of cyclin-dependent kinases to prevent cells from entering the S phase. In addition, the Notch signaling pathway inhibits cell differentiation by activating specific downstream target genes, such as the Hes family and the Hey family, and this inhibition is essential for maintaining the correct ratio of inner ear hair cells to supporting cells [33].
In addition, Math1 and Hesl are important regulators that promote hair cell differentiation. It was found that in mice, if Math1 was knocked out, embryonic mice did have cochlear and vestibular hair cells, and vice versa, regeneration of cochlear hair cells and differentiation of vestibular hair cells were observed [34,35]. Hesl, in contrast to Math1, is a negative regulator in cochlear development, and both play an important role in normal cochlear development [36,37].
Extracellular environmental
The extracellular microenvironment, including the extracellular matrix (ECM), surrounding cells, solubility factors (e.g., growth factors and cytokines), as well as physical and chemical conditions (e.g., oxygen concentration, stiffness, and pH), also have a profound effect on the proliferation and differentiation of inner ear cells [38].
The extracellular matrix is (ECM) a complex network of multiple proteins and polysaccharides that not only provides physical support for the cell, but is also involved in regulating cell behavior [39,40]. In the inner ear, components of the extracellular matrix (ECM) such as fibronectin, laminin and collagen can influence the adhesion, proliferation and differentiation of inner ear stem cells [41,42]. Laminin (LN) is a heterotrimer of α, β and γ-chains, which is located in the basement membrane and subbasement membrane surrounding the spiral ganglion of the mammalian cochlea. The dimeric glycoprotein fibronectin (FN) has also been found to be located in the cochlea [43], both of them grow alongside spiral ganglion dendrite-targeted hair cells during cochlear maturation and subsequently influence cochlear hair cell growth and differentiation. In 2005, Tomama et al., identified in the cochlea of perinatal mammals heterodimers of the integrin ECM receptor family, which was previously found to be expressed in the developing brain and consists of a heterodimeric transmembrane protein consisting of an α chain and a β chain, which binds to a variety of molecules in the ECM, linking them to the actin cytoskeleton and other intracellular effectors [42,44]. Specific ECM components can activate specific signaling pathways, such as FGF, Wnt/β-catenin, etc., which in turn affects the direction of cell differentiation.
Peripheral cells in the inner ear microenvironment are also important for the proliferation and differentiation of inner ear stem cells, and they can regulate each other through direct contact with specific receptors and ligands (intercellular adhesions) on the cell surface, or interact with each other through, for example, the secretion of solubility factors. Yushi et al. [45], found that cochlear support cells protect the survival and function of hair cells by inducing type I interferon after viral infection, in addition, damage or death of ciliated cells can activate surrounding support cells, inducing them to transform into stem cells and participate in repair and regeneration processes. The nerve cells of the cochlear, vestibular and spiral nerves in the inner ear can influence the neural differentiation of stem cells through direct contact or secretion of nerve growth factors, etc. Fibroblasts are the main synthesizing cells of the extracellular matrix (ECM), which can influence the attachment/ proliferation and differentiation of stem cells by regulating the composition of the extracellular matrix, and also influence the proliferation and transformation to specific types of cells by secretion of fibroblast growth factors. Proliferation and transformation of inner ear stem cells to specific cell types through the secretion of fibroblast growth factors [46]. In addition, fibroblasts are involved in local immune responses and inflammatory processes, which can affect the microenvironment of inner ear stem cells through the secretion of cytokines and chemokines, thereby influencing their value-added and differentiation [41,47].
Soluble factors in the extracellular matrix mainly include epidermal growth factor (EGF), fibroblast growth factor (FGF), nerve growth factor, transforming growth factor-β (TGF-β), insulin-like growth factor and estrogen (IGF-1), prostaglandin E2, retinol, tumor necrosis factor alpha (TNF-alpha), and interleukins (IL-1 and IL-6). They are all proteins or peptides produced by cells that have the ability to influence cell behavior. These molecules can affect cell proliferation, differentiation, migration, etc. by binding to specific cell surface receptors or specific signaling pathways. Campbell et al. [48], found that epidermal growth factor (EGF) and fibroblast growth factor (FGF), could promote the proliferation and differentiation of neonatal mouse neural stem cells. Lian M et al.[49], found through in vitro cellular experiments that nerve growth factor could promote the functional and neurotrophic effects of bone marrow mesenchymal stem cells, which improved the osteogenic capacity of bone repair cells. Chen G et al. [50], injected transforming growth factor-β-1-treated extracellular vesicles (T-EVS) into spinal cord-injured mice and found that it significantly enhanced the proliferation and anti-apoptotic capacity of neural stem cells in vitro, as well as increased the transition of reactive microglial cells from M1 to M2, which resulted in attenuation of neuroinflammation and enhancement of neuroprotective effects of residual cells in the acute phase. In neonatal mammals with different types of hair cell injury, insulin- like growth factor-1 (IGF-1) maintains the number of hair cells in the cochlea by activating two major pathways downstream of the IGF-1 signaling pathway, and in aminoglycoside-treated neonatal mouse cochlear explant cultures, the IGF-1-treated group promotes support for the cell cycle and inhibits apoptosis of capillary cells [51]. Poletti V et al. [52], found that prostaglandin E2 increased the transduction efficiency of hematopoietic stem cell progeny in vitro. Vitamin A (including retinol and its active form retinoic acid) plays an important role in the regulation of stem cell fate determination and cell lineage plasticity, and retinoic acid serves as an important signaling molecule that can directly regulate the transcription of target genes by binding to intracellular retinoic acid receptors. This mechanism allows vitamin A to precisely regulate the differentiation process of stem cells, including promoting the formation of certain cell types and inhibiting the formation of other [53].Tumor necrosis factor (TNF-α) is a cytokine that is produced mainly by immune cells and is widely involved in inflammatory responses, immune regulation, and apoptotic and survival processes. In the study of inner ear stem cells, the role of TNF-α has also attracted attention, especially its potential effects on the proliferation and differentiation of inner ear stem cells, which may be promoted or inhibited through the activation of signaling pathways such as NF-κB, or through the alteration of intracellular signaling networks and the regulation of the expression of specific genes. One study knocked out the tumor necrosis factor gene receptor from lung cancer mouse cells, resulting in a significant reduction in tumor size and weight in TNFR2 knockout mice compared to wild-type mice [54]. Interleukins (ILs) are an important class of cytokines widely involved in immune regulation, cell proliferation, differentiation, and inflammatory responses. One research found that the expression of interleukin 1β and insulin- like growth factor-1 was up-regulated in neurons and glial cells of the cochlear ventral nucleus in adult rats at 1, 7, and 15 days after bilateral cochlear surgery, reflecting the possible involvement of IL-1 in repairing the synaptic dynamic balance of the overall cellular environment of the cochlear nucleus [55]. IL-2, IL-4, IL-6, and IL-8 promote the proliferation and differentiation of certain inner ear cells by activating the JAK/STAT signaling pathway or nuclear factor-κB (NF-κB) pathway, etc., which promote the proliferation and differentiation of certain inner ear cells [56,57].
The proliferation and differentiation of inner ear stem cells are regulated by a combination of genetic, environmental and biochemical factors. A deeper understanding of these influencing factors will not only help us to reveal the repair mechanisms after inner ear injury, but will also provide a scientific basis for the development of new therapeutic strategies. Future studies need to explore in greater depth how these factors interact with each other and how to promote inner ear regeneration and repair by interfering with these factors, thus opening up new avenues for the treatment of hearing loss and balance dysfunction.
REFERENCES
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003; 9: 1293-1299.
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Géléoc GS, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007; 8: 18-31.
- Zhang Y, Zhai SQ, Shou J, Song W, Sun JH, Guo W, et al. Isolation, growth and differentiation of hair cell progenitors from the newborn rat cochlear greater epithelial ridge. J Neurosci Methods. 2007; 164: 271-279.
- Li H, Kloosterman W, Fekete DM. MicroRNA-183 family members regulate sensorineural fates in the inner ear. J Neurosci. 2010; 30: 3254-3263.
- Mahmoodian Sani MR, Hashemzadeh-Chaleshtori M, Saidijam M, Jami MS, Ghasemi-Dehkordi P. MicroRNA-183 Family in Inner Ear: Hair Cell Development and Deafness. J Audiol Otol. 2016; 20: 131-138.
- Lewis MA, Di Domenico F, Ingham NJ, Prosser HM, Steel KP. Hearing impairment due to Mir183/96/182 mutations suggests both loss and gain of function effects. Dis Model Mech. 2020; 14: dmm047225.
- Geng R, Furness DN, Muraleedharan CK, Zhang J, Dabdoub A, Lin V, et al. The microRNA-183/96/182 Cluster is Essential for Stereociliary Bundle Formation and Function of Cochlear Sensory Hair Cells. Sci Rep. 2018; 8: 18022.
- Zhou W, Du J, Jiang D, Wang X, Chen K, Tang H, et al. microRNA 183 is involved in the differentiation and regeneration of Notch signaling prohibited hair cells from mouse cochlea. Mol Med Rep. 2018; 18: 1253-1262.
- Niu Z, Goodyear SM, Rao S, Wu X, Tobias JW, Avarbock MR, et al. MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2011; 108: 12740-12745.
- Hao F, Shan C, Zhang Y, Zhang Y, Jia Z. Exosomes Derived from microRNA-21 Overexpressing Neural Progenitor Cells Prevent Hearing Loss from Ischemia-Reperfusion Injury in Mice via Inhibiting the Inflammatory Process in the Cochlea. ACS Chem Neurosci. 2022; 13: 2464-2472.
- Chawra HS, Agarwal M, Mishra A, Chandel SS, Singh RP, Dubey G, et al. MicroRNA-21’s role in PTEN suppression and PI3K/AKT activation: Implications for cancer biology. Pathol Res Pract. 2024; 254: 155091.
- Zhang R, Sun Y, Zhang Q, Lin J, Zhang Y, Chen X, et al. Overexpression of miR-124-3p affects zebrafish inner ear development and hearing function via downregulation of EYA1 gene expression. Neurosci Lett. 2023; 802: 137172.
- Jiang D, Du J, Zhang X, Zhou W, Zong L, Dong C, et al. miR-124 promotes the neuronal differentiation of mouse inner ear neural stem cells. Int J Mol Med. 2016; 38: 1367-1376.
- Xiong H, Pang J, Yang H, Dai M, Liu Y, Ou Y, et al. Activation of miR- 34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis: implications for age-related hearing loss. Neurobiol Aging. 2015; 36: 1692-1701.
- Wang Y, Zhao J, Chen S, Li D, Yang J, Zhao X, et al. Let-7 as a Promising Target in Aging and Aging-Related Diseases: A Promise or a Pledge. Biomolecules. 2022; 12: 1070.
- Kuan II, Lee CC, Chen CH, Lu J, Kuo YS, Wu HC. The extracellular domain of epithelial cell adhesion molecule (EpCAM) enhances multipotency of mesenchymal stem cells through EGFR-LIN28-LET7 signaling. J Biol Chem. 2019; 294: 7769-7786.
- Ye Z, Su Z, Xie S, Liu Y, Wang Y, Xu X, et al. Yap-lin28a axis targets let7- Wnt pathway to restore progenitors for initiating regeneration. Elife. 2020; 9: e55771.
- Di Stadio A, Pegoraro V, Giaretta L, Dipietro L, Marozzo R, Angelini C. Hearing impairment in MELAS: new prospective in clinical use of microRNA, a systematic review. Orphanet J Rare Dis. 2018; 13: 35.
- Moutabian H, Radi UK, Saleman AY, Adil M, Zabibah RS, Chaitanya MNL, Saadh MJ, Jawad MJ, Hazrati E, Bagheri H, Pal RS, Akhavan-Sigari R. MicroRNA-155 and cancer metastasis: Regulation of invasion, migration, and epithelial-to-mesenchymal transition. Pathol Res Pract. 2023; 250: 154789.
- Hongmiao R, Wei L, Bing H, Xiong DD, Jihao R. Atoh1: landscape for inner ear cell regeneration. Curr Gene Ther. 2014; 14: 101-111.
- Choi SW, Abitbol JM, Cheng AG. Hair Cell Regeneration: From Animals to Humans. Clin Exp Otorhinolaryngol. 2024; 17: 1-14.
- Zhang YL, Kang M, Wu JC, Xie MY, Xue RY, Tang Q, et al. Small activating RNA activation of ATOH1 promotes regeneration of human inner ear hair cells. Bioengineered. 2022; 13: 6729-6739.
- McGovern MM, Hosamani IV, Niu Y, Nguyen KY, Zong C, Groves AK. Expression of Atoh1, Gfi1, and Pou4f3 in the mature cochlea reprograms nonsensory cells into hair cells. Proc Natl Acad Sci U S A. 2024; 121: e2304680121.
- Li X, Ren M, Gu Y, Zhu T, Zhang Y, Li J, et al. In situ regeneration of inner hair cells in the damaged cochlea by temporally regulated co- expression of Atoh1 and Tbx2. Development. 2023; 150: dev201888.
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006; 133: 865-875.
- Hosseini V, Dani C, Geranmayeh MH, Mohammadzadeh F, Nazari Soltan Ahmad S, Darabi M. Wnt lipidation: Roles in trafficking, modulation, and function. J Cell Physiol. 2019; 234: 8040-8054.
- Shi F, Hu L, Jacques BE, Mulvaney JF, Dabdoub A, Edge AS. β-Catenin is required for hair-cell differentiation in the cochlea. J Neurosci. 2014; 34: 6470-6479.
- Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012; 109: 8167-8172.
- Kuo BR, Baldwin EM, Layman WS, Taketo MM, Zuo J. In Vivo Cochlear Hair Cell Generation and Survival by Coactivation of β-Catenin and Atoh1. J Neurosci. 2015; 35: 10786-98.
- Gozlan O, Sprinzak D. Notch signaling in development and homeostasis. Development. 2023; 150: dev201138.
- Shu Y, Li W, Huang M, Quan YZ, Scheffer D, Tian C, et al. Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat Commun. 2019; 10: 5530.
- Brown R, Groves AK. Hear, Hear for Notch: Control of Cell Fates in the Inner Ear by Notch Signaling. Biomolecules. 2020; 10: 370.
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999; 284: 1837-1841.
- Li S, Qian W, Jiang G, Ma Y. Transcription factors in the development of inner ear hair cells. Front Biosci (Landmark Ed). 2016; 21: 1118- 1125.
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, et al. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001; 21: 4712- 4720.
- You D, Ni W, Huang Y, Zhou Q, Zhang Y, Jiang T, et al. The proper timing of Atoh1 expression is pivotal for hair cell subtype differentiation and the establishment of inner ear function. Cell Mol Life Sci. 2023; 80: 349.
- Pressé MT, Malgrange B, Delacroix L. The cochlear matrisome: Importance in hearing and deafness. Matrix Biol. 2024; 125: 40-58.
- Goh SK, Halfter W, Richardson T, Bertera S, Vaidya V, Candiello J, et al. Organ-specific ECM arrays for investigating cell-ECM interactions during stem cell differentiation. Biofabrication. 2020; 13.
- Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020; 584: 535-546.
- Zhang J, Liu L, Li Y, Wu J, Lou X. Mouse Embryonic Fibroblasts-Derived Extracellular Matrix Facilitates Expansion of Inner Ear-Derived Cells. Cell J. 2023; 25: 447-454.
- Evans AR, Euteneuer S, Chavez E, Mullen LM, Hui EE, Bhatia SN, et al. Laminin and fibronectin modulate inner ear spiral ganglion neurite outgrowth in an in vitro alternate choice assay. Dev Neurobiol. 2007; 67: 1721-1730.
- Cosgrove D, Rodgers KD. Expression of the major basement membrane-associated proteins during postnatal development in the murine cochlea. Hear Res. 1997; 105: 159-170.
- Johnson Chacko L, Lahlou H, Steinacher C, Assou S, Messat Y, Dudás J, et al. Transcriptome-Wide Analysis Reveals a Role for Extracellular Matrix and Integrin Receptor Genes in Otic Neurosensory Differentiation from Human iPSCs. Int J Mol Sci. 2021; 22: 10849.
- Hayashi Y. Signaling pathways regulating the immune function of cochlear supporting cells and their involvement in cochlear pathophysiology. Glia. 2024; 72: 665-676.
- Yang Q, Shi H, Quan Y, Chen Q, Li W, Wang L, et al. Stepwise Induction of Inner Ear Hair Cells From Mouse Embryonic Fibroblasts via Mesenchymal to Epithelial Transition and Formation of Otic Epithelial Cells. Front Cell Dev Biol. 2021; 9: 672406.
- Cumpata AJ, Labusca L, Radulescu LM. Stem Cell-Based Therapies for Auditory Hair Cell Regeneration in the Treatment of Hearing Loss. Tissue Eng Part B Rev. 2024; 30: 15-28.
- Campbell CE, Webber K, Bard JE, Chaves LD, Osinski JM, Gronostajski RM. Nuclear Factor I A and Nuclear Factor I B Are Jointly Required for Mouse Postnatal Neural Stem Cell Self-Renewal. Stem Cells Dev. 2024; 33: 153-167.
- Lian M, Qiao Z, Qiao S, Zhang X, Lin J, Xu R, et al. Nerve Growth Factor-Preconditioned Mesenchymal Stem Cell-Derived Exosome- Functionalized 3D-Printed Hierarchical Porous Scaffolds with Neuro- Promotive Properties for Enhancing Innervated Bone Regeneration. ACS Nano. 2024; 18: 7504-7520.
- Chen G, Tong K, Li S, Huang Z, Liu S, Zhu H, et al. Extracellular vesicles released by transforming growth factor-beta 1-preconditional mesenchymal stem cells promote recovery in mice with spinal cord injury. Bioact Mater. 2024; 35: 135-149.
- Yamamoto N, Nakagawa T, Ito J. Application of insulin-like growth factor-1 in the treatment of inner ear disorders. Front Pharmacol. 2014; 5: 208.
- Poletti V, Montepeloso A, Pellin D, Biffi A. Prostaglandin E2 as transduction enhancer affects competitive engraftment of human hematopoietic stem and progenitor cells. Mol Ther Methods Clin Dev. 2023; 31: 101131.
- Tierney MT, Polak L, Yang Y, Abdusselamoglu MD, Baek I, Stewart KS, Fuchs E. Vitamin A resolves lineage plasticity to orchestrate stem cell lineage choices. Science. 2024; 383: eadi7342.
- Yeo IJ, Yu JE, Kim SH, Kim DH, Jo M, Son DJ, et al. TNF receptor 2 knockout mouse had reduced lung cancer growth and schizophrenia- like behavior through a decrease in TrkB-dependent BDNF level. Arch Pharm Res. 2024; 47: 341-359.
- Fuentes-Santamaría V, Alvarado JC, Gabaldón-Ull MC, Manuel Juiz J. Upregulation of insulin-like growth factor and interleukin 1β occurs in neurons but not in glial cells in the cochlear nucleus following cochlear ablation. J Comp Neurol. 2013; 521: 3478-3499.
- Kubo T, Anniko M, Stenqvist M, Hsu W. Interleukin-2 affects cochlear function gradually but reversibly. ORL J Otorhinolaryngol Relat Spec. 1998; 60: 272-277.
- Tan CQ, Gao X, Guo L, Huang H. Exogenous IL-4-expressing bone marrow mesenchymal stem cells for the treatment of autoimmune sensorineural hearing loss in a guinea pig model. Biomed Res Int. 2014; 2014: 856019.
- Iguchi H, Anniko M. Interleukin 8 can affect inner ear function. ORL J Otorhinolaryngol Relat Spec. 1998; 60: 181-189.