Meta-Analysis of Gerd-Related Outcomes Following Roux-En-Y Gastric Bypass Versus One Anastomosis Gastric Bypass: Evidence against the Incautious use of OAGB in Patients with Pre-Existing Gerd
- 1. North Midlands Upper GI & Bariatric Unit, University Hospitals North Midlands NHS Trust, United Kingdom
Abstract
Objectives : To evaluate GERD-related outcomes following Roux-en-Y gastric bypass (RYGB) versus One Anastomosis Gastric Bypass (OAGB) in both primary and revisional bariatric surgeries, with the objective of determining whether RYGB offers superior outcomes and should be preferred over OAGB in patients with established GERD.
Methods: A systematic search of Medline, Embase, CINAHL, CENTRAL, Web of Science, and relevant registries was performed. A random-effects meta-analysis was undertaken using PRISMA guidelines to assess GERD remission, de novo GERD, GERD incidence, and endoscopically confirmed oesophagitis between RYGB and OAGB.
Results :Twenty-one observational studies with a total of 4, 143 patients were included. GERD remission was more likely following RYGB compared to OAGB in both primary (OR = 3.18, 95% CI = [0.70, 14.51], P = 0.14) and revisional surgery (OR = 3.30, 95% CI = [0.77, 14.20], P = 0.11). De novo GERD was significantly less likely after RYGB in both primary (OR = 0.43, 95% CI = [0.16, 1.11], P = 0.08) and revisional settings (OR = 0.08, 95% CI = [0.01, 0.44], P = 0.004). Overall GERD incidence significantly favoured RYGB in both primary (OR = 0.33, 95% CI = [0.16, 0.66], P = 0.002) and revisional cases (OR = 0.27, 95% CI = [0.09, 0.81], P = 0.02). Endoscopically proven oesophagitis was less frequent after RYGB in both primary (OR = 0.57, 95% CI = [0.25, 1.28], P = 0.17) and revisional surgery (OR = 0.30, 95% CI = [0.05, 1.69], P = 0.17.
Conclusions :OAGB is associated with higher rates of de novo GERD, increased GERD incidence, and more frequent endoscopic evidence of oesophagitis when compared to RYGB. These findings suggest that OAGB should be used with caution in patients known GERD or to be considered with a close postoperative surveillance.
Keywords: GERD; Roux-en-Y Gastric Bypass; One Anastomosis Gastric Bypass; Meta-analysis; Bariatric Surgery
Introduction
Bariatric surgery has emerged as a highly effective intervention for individuals with morbid obesity, with Roux-en-Y gastric bypass (RYGB) and one anastomosis gastric bypass (OAGB) being among the most widely performed procedures [1,2]. As global obesity rates continue to rise, the growing number of bariatric surgeries has led to an increasing population of patients requiring postoperative evaluation for gastroesophageal reflux disease (GERD) [3]. Both RYGB and OAGB are effective in achieving significant weight loss and metabolic improvements; however, they differ considerably in their impact on GERD [3-5]. RYGB mitigates GERD through the creation of a small gastric pouch and the diversion of gastric contents, thereby reducing gastric volume, pressure, and acid exposure in the oesophagus. In contrast, OAGB—a simplified variant of gastric bypass—has gained popularity due to its technical ease and comparable weight loss outcomes. Nevertheless, concerns persist regarding the potential for increased incidence of GERD following OAGB, particularly among patients with pre-existing reflux disease [6,7]. A clear understanding of the reflux-related outcomes associated with these procedures is essential for informed surgical decision-making. We aimed to conduct a comprehensive systematic review and meta-analysis of the best available evidence comparing RYGB and OAGB with respect to GERD-related outcomes, including GERD remission, de novo GERD, overall GERD incidence, and postoperative endoscopically confirmed esophagitis. The findings aim to provide clinically relevant insights into the suitability of OAGB, especially for patients with pre-existing GERD. Vol :(0123456789)
Methods
Design and Study Selection
The inclusion and exclusion criteria, methodology, and investigated outcome parameters of this review were highlighted in a review protocol. Our methodology, consistent with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [8].
Inclusion Criteria
•All studies comparing GERD-related outcomes between RYGB and OAGB
•Including any adult patients (≥18 years) undergoing primary or revisional procedures
•At least one GERD outcome reported: remission, de novo GERD, incidence, or endoscopic findings
Exclusion Criteria
•Case reports, expert opinions, letters, or editorials
•Studies with less than 12 months of follow-up
Outcomes
The primary outcome parameters were GERD remission, de novo GERD, overall GERD incidence, and post-operative endoscopically confirmed oesophagitis.
Literature Search Strategy
A comprehensive search strategy was formulated based on thesaurus headings, search operators, and limits in Medline, Embase, CINAHL, CENTRAL, and Web of Science. A literature search was conducted via the aforementioned databases and also searched World Health Organization International Clinical Trials Registry http:// apps. who. int/ trial search/, ClinicalTrials.gov http://c linicaltri als .gov /, and ISRCTN Register http:// www. isrctn. com/ to identify ongoing and unpublished studies. Moreover, the reference lists were searched of relevant articles and reviews to identify relevant trials.
Selection of Studies
The title and abstract of articles found as the result of the literature search were carefully assessed. When necessary, the full texts of relevant articles were retrieved and carefully evaluated against the eligibility criteria of this study. Studies that met the eligibility criteria were included.
Data Extraction and Management
According to the Cochrane’s recommendations for intervention reviews, an electronic data extraction spreadsheet was created and was pilot-tested in randomly selected articles and adjusted accordingly. The following information was extracted from each of the included studies:
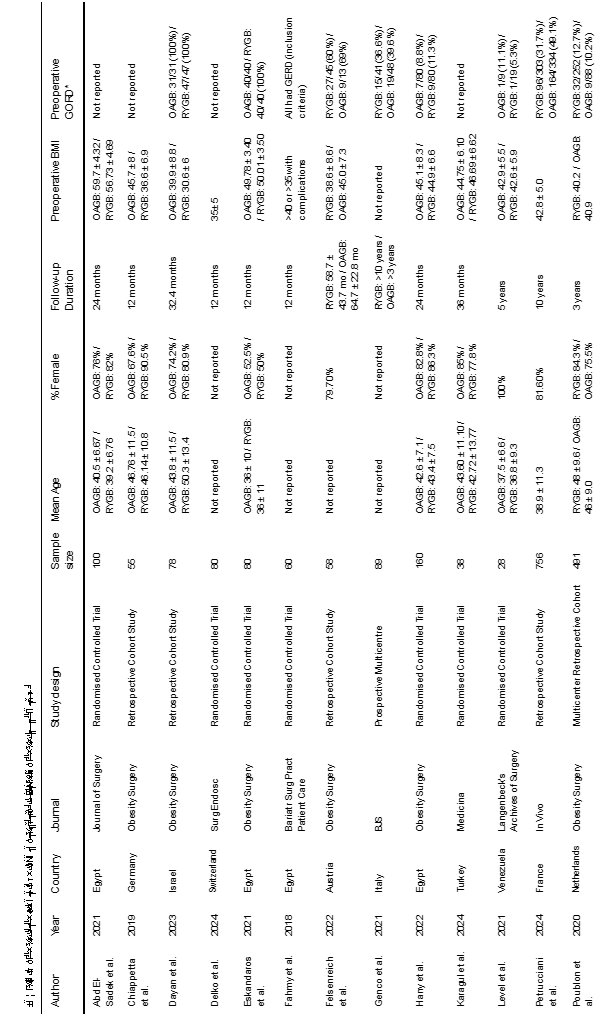
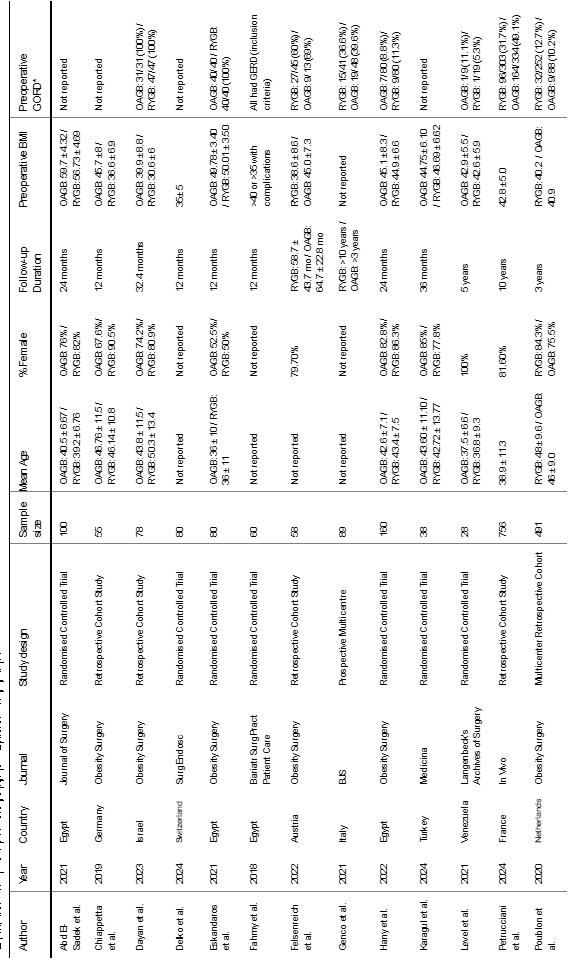
•Study-related data (first author, publication year, country of origin of the corresponding author, journal in which the study was published, study design, procedure performed, and sample size)
•Baseline demographic and clinical information of the study populations (age, gender, body mass index (BMI), preoperative GORD and follow-up period)
•Outcome data
Assessment of Risk of Bias
The methodological quality and risk of bias assessment were carried out using the Newcastle–Ottawa scale (NOS) [9], as all of our included studies were observational studies. The NOS is a star-based scoring system (maximum score: 9) which enables review authors to evaluate an observational study in the following aspects: the selection of the study groups, the comparability of the groups, and the ascertainment of outcome of interest. Studies with a score of nine stars were deemed to be at low risk of bias, studies with a score of seven or eight stars were deemed to be at medium risk of bias, and those that scored six or less were judged to be at high risk of bias.
Summary Measures and Synthesis
Cochrane software was used for the proportion meta-analysis model. We integrated the quantitative risk of GERD remission, De novo GERD, Overall GERD incidence, Post Op endoscopically proven oesophagitis from individual studies and calculated a numerical estimates of the overall effect. The DerSimonoan-Laird random-effects method was used to calculate the weighted summary proportions under the random effects modelling. Intention to treat information data from the included studies were used for data analysis, and individual patients were considered the unit of analysis. Heterogeneity among the studies was assessed using the Cochran Q test (χ2) [10]. We quantified inconsistency by calculating I2 and interpreted it using the following guide: 0 to 40% might not be important; 30 to 60% may represent moderate heterogeneity; 50 to 90% may represent substantial heterogeneity, and 75 to 100% may represent considerable heterogeneity.
Results
Our literature search resulted in 2146 articles. After further evaluation of the identified articles, 49 articles were shortlisted for potential inclusion. A further 11 studies were excluded as they did not involve the appropriate intervention or comparator. Additionally, 9 studies were excluded as they did not provide a direct comparison between OAGB and RYGB. Five studies were excluded due to a follow-up period of less than 12 months. Moreover, another 3 studies were excluded as they were earlier reports of studies already included in the analysis. Finally, 21 observational studies [7,11-30], were deemed appropriate for inclusion (Figure 1). The included studies reported a total number of 4143 patients who underwent RYGB or OAGB and were followed up for at least 12 months. Table 1 presents the date of publication and country of origin, journal, study design of the included studies, sample sizes, and baseline characteristics of the study populations The median follow-up period was 32.4 months (range: 12–120). The median pre-operative GERD prevalence was 15.0% in the OAGB group and 34.15% in the RYGB group.
Methodological Appraisal
The methodological appraisal of all included observational studies is presented in Table 1 and Figure 2. The risk of bias was judged as low in 9 studies and moderate in 12 studies.
Outcome Data
Outcomes are summarised in Figures 3-6.
GERD remission
Analysis of 686 patients from 10 studies demonstrated that the odds of GERD remission were significantly higher after RYGB compared to OAGB, with a pooled odds ratio of 3.33 (95% CI 1.22–9.08, P = 0.02). The between-study heterogeneity was substantial (I² = 74%, P < 0.001) (Figure 3).
Subgroup analysis of primary surgery patients (4 studies; 328 patients), RYGB was associated with a non-significant trend toward higher GERD remission compared to OAGB (OR 3.18, 95% CI 0.70–14.51, P = 0.14), with moderate heterogeneity (I² = 53%).
In the revision post-sleeve gastrectomy group (6 studies; 358 patients), RYGB was associated with a non-significant trend toward higher GERD remission compared to OAGB (OR 3.30, 95% CI 0.77–14.20, P = 0.11), but this subgroup exhibited high heterogeneity (I² = 79%). No significant difference was observed between subgroups (P = 0.97), suggesting consistency of the effect across surgical contexts
Overall GERD incidence
Analysis of 2,502 patients from 12 studies demonstrated that the odds of developing GERD were significantly lower after RYGB compared to OAGB, with a pooled odds ratio of 0.32 (95% CI 0.18–0.58, P = 0.0002). The between-study heterogeneity was moderate (I² = 49%, P = 0.01) (Figure 4).
Subgroup analysis of primary surgery patients (9 studies; 2,256 patients) showed significantly lower GERD incidence in the RYGB group compared to the OAGB group (OR 0.33, 95% CI 0.16–0.66, P = 0.002), with moderate heterogeneity (I² = 58%).
In the revision post-sleeve gastrectomy group (3 studies; 246 patients), RYGB also showed significantly lower GERD incidence than OAGB (OR 0.27, 95% CI 0.09–0.81, P = 0.02), with no heterogeneity (I² = 0%).
No significant difference was observed between subgroups (P = 0.76), suggesting that the protective effect of RYGB over OAGB with regard to GERD incidence was consistent across surgical contexts.
De Novo GER
Analysis of 1,323 patients from 11 studies demonstrated that the odds of developing de novo GERD were significantly lower after RYGB compared to OAGB, with a pooled odds ratio of 0.30 (95% CI 0.12–0.74, P = 0.009). The between-study heterogeneity was moderate (I² = 55%, P = 0.008) (Figure 5).
Subgroup analysis of primary surgery patients (8 studies; 890 patients) revealed a non-significant trend toward lower risk of de novo GERD with RYGB compared to OAGB (OR 0.43, 95% CI 0.16–1.11, P = 0.08), with moderate heterogeneity (I² = 55%).
In contrast, in the revision post-sleeve gastrectomy group (5 studies; 433 patients), RYGB was associated with significantly lower odds of developing de novo GERD compared to OAGB (OR 0.08, 95% CI 0.01–0.44, P = 0.004), with no observed heterogeneity (I² = 0%).
The difference between subgroups was not statistically significant (P = 0.09), suggesting that the protective effect of RYGB may be more pronounced after revision surgery but not conclusively different between subgroups.
Post-operative endoscopically Proven Oesophagitis
Analysis of 550 patients from 6 studies demonstrated a non-significant trend toward lower odds of endoscopically confirmed oesophagitis after RYGB compared to OAGB, with a pooled odds ratio of 0.53 (95% CI 0.26–1.08, P = 0.08). The between-study heterogeneity was low (I² = 34%, P = 0.23) (Figure 6).
Subgroup analysis of primary surgery patients (5 studies; 510 patients) also showed a non-significant reduction in oesophagitis incidence after RYGB (OR 0.57, 95% CI 0.25–1.28, P = 0.17), with moderate heterogeneity (I² = 41%).
In the revision post-sleeve gastrectomy group (1 study; 40 patients), RYGB was again associated with a non-significant trend toward reduced oesophagitis compared to OAGB (OR 0.30, 95% CI 0.05–1.69, P = 0.17), with no applicable heterogeneity due to a single study.
No significant difference was observed between subgroups (P = 0.50), suggesting consistency in the trend across surgical contexts.
Figure 1
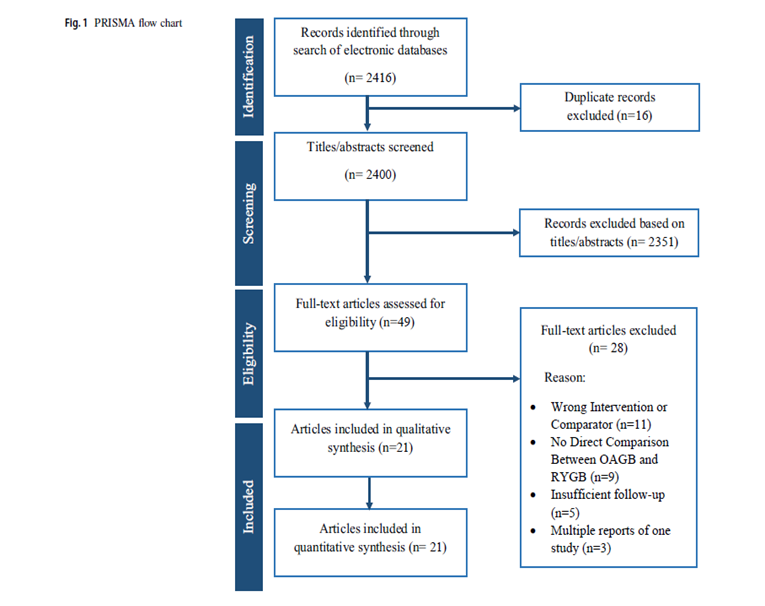
Figure 1: Prisma Flow Chart
Figure 2
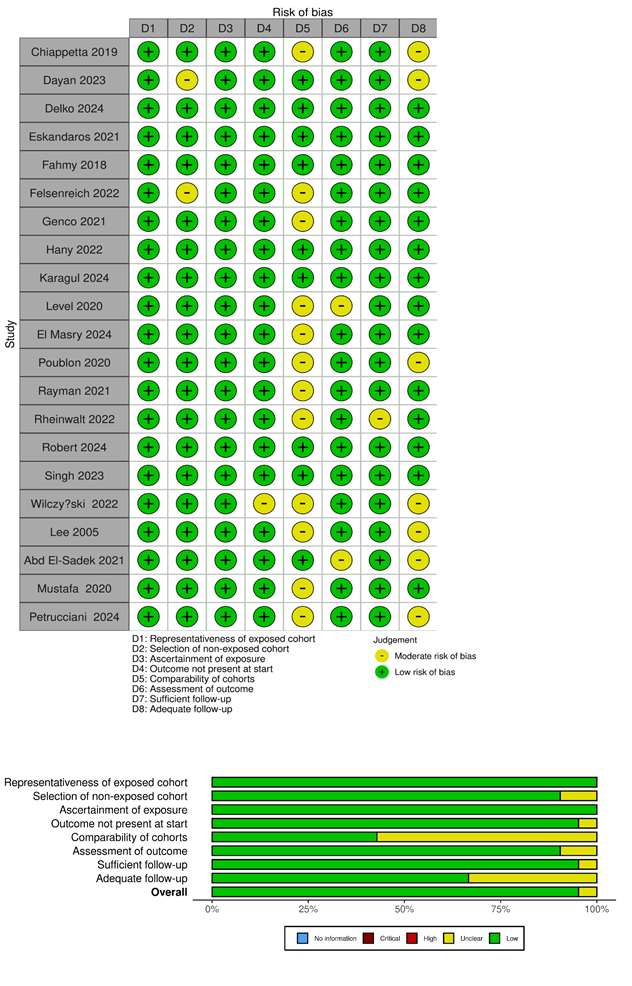
Figure 2: Risk of bias summary and a graph showing authors’ judgments about each risk of bias item
Figure 3
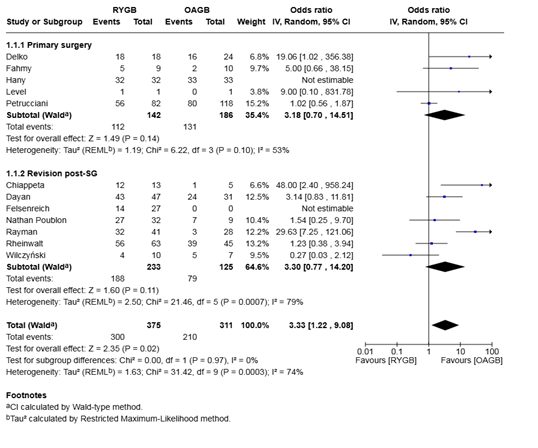
Figure 3: Forest plot for the proportion meta-analysis of the outcomes after surgery showing the pooled rate of GERD remission
Figure 4
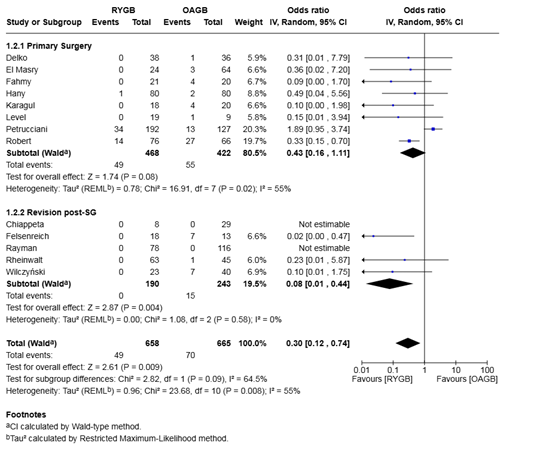
Figure 4: Forest plot for the proportion meta-analysis of the outcomes after surgery showing the estimated proportion of patients who developed de novo GERD.
Figure 5
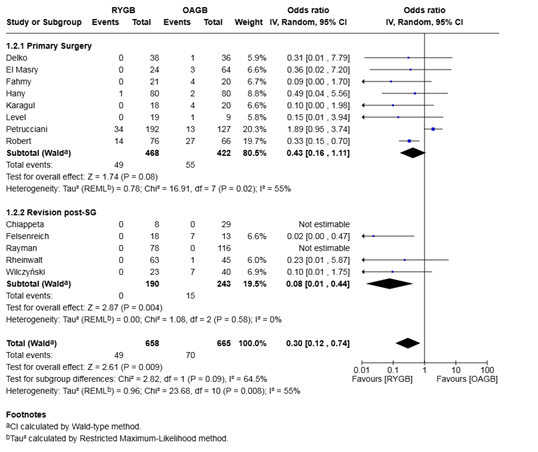
Figure 5: Forest plot for the proportion meta-analysis of the outcomes after surgery showing the overall incidence of post-operative GERD.
Figure 6
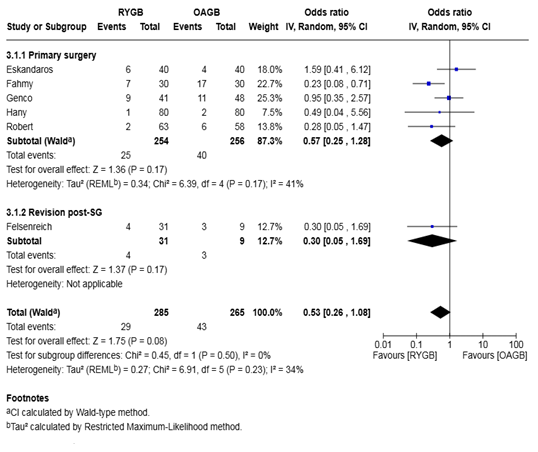
Figure 6: Forest plot for the proportion meta-analysis of the outcomes after surgery showing the pooled proportion of patients with endoscopically confirmed post-operative oesophagitis.
Discussion
In view of the ongoing debate regarding the optimal bariatric procedure for patients at risk of gastroesophageal reflux disease (GERD), we conducted a comprehensive systematic review and meta-analysis to compare the reflux-related outcomes of Roux-en-Y gastric bypass (RYGB) and one-anastomosis gastric bypass (OAGB). A total of 21 observational studies encompassing 4,143 patients who underwent either RYGB or OAGB were included. These studies comprised both primary and revisional bariatric procedures, with adequate follow-up data on GERD-related outcomes. The pooled analysis revealed that RYGB was associated with a significantly higher likelihood of GERD remission compared to OAGB, with a pooled odds ratio of 3.33 (95% CI 1.22–9.08, P = 0.02). However, the considerable between-study heterogeneity (I² = 74%) limits the robustness of this finding and likely reflects variation in surgical techniques, diagnostic criteria for GERD, and baseline patient characteristics. Subgroup analyses of primary and revisional procedures demonstrated non-significant trends favoring RYGB, suggesting a potential benefit while highlighting the need for more uniform data across studies. GERD affects approximately 10-30% of the general adult population in developed countries and occurs at a higher rate among individuals with obesity, with reported prevalence ranging from 22% to 70%. Our findings indicate that the risk of de novo GERD—defined as new-onset GERD following surgery-was significantly lower following RYGB compared to OAGB (OR 0.30, 95% CI 0.12–0.74, P = 0.009). This protective effect was particularly pronounced in the revisional post-sleeve gastrectomy subgroup, where the odds ratio decreased to 0.08 (95% CI 0.01–0.44, P = 0.004), with no observed heterogeneity (I² = 0%), indicating a robust association. Although the primary surgery subgroup did not achieve statistical significance (OR 0.43, P = 0.08), the consistent direction of effect across subgroups supports a reflux-mitigating role for RYGB. The analysis of endoscopically confirmed postoperative oesophagitis suggested a non-significant trend toward lower incidence following RYGB compared to OAGB (OR 0.53, P = 0.08). Subgroup analyses similarly failed to reach statistical significance. The limited number of studies reporting on this outcome and relatively small sample sizes may have resulted in underpowered estimates. Additionally, the absence of reported oesophagitis in several studies raises concerns about selective outcome reporting. As noted in prior reflux-related meta-analyses, underreporting of negative outcomes may lead to inflated rates among studies that do report, thereby distorting true prevalence estimates. The clinical implications of these findings are considerable. For patients with pre-existing GERD or those considered at elevated risk for reflux-related complications, RYGB appears to offer a more favorable profile compared to OAGB. This advantage is likely attributable to the anatomical modifications inherent to RYGB, which involve creating a small gastric pouch and diverting gastric contents away from the esophagus. The Roux-en-Y configuration reduces intragastric pressure and volume while bypassing bile flow, thus minimizing acid and bile exposure to the lower esophagus [31]. Furthermore, bariatric surgery in general confers metabolic benefits beyond weight loss, including improvements in type 2 diabetes, hypertension, and dyslipidemia [32]. Despite these benefits, RYGB carries procedure-specific risks, including anastomotic leaks, internal hernias, and micronutrient deficiencies, all of which necessitate structured postoperative monitoring and supplementation [33,34] This meta-analysis also highlights several limitations in the current evidence base. Most included studies were retrospective observational cohorts, making them inherently susceptible to selection bias, confounding, and inconsistent follow-up. Variability in GERD diagnostic methods—ranging from symptom-based questionnaires to objective endoscopy or pH monitoring—further contributed to heterogeneity. Additionally, the lack of standardized outcome definitions and underreporting of negative findings limits the generalizability and precision of our estimates. Nevertheless, this study provides a timely and clinically relevant synthesis of existing evidence that may aid surgical decision-making, particularly in patients at risk of reflux. Future high-quality prospective studies with standardized GERD definitions, objective diagnostic criteria, and long-term follow-up are essential to validate these findings and inform guideline development. Randomized controlled trials comparing RYGB and OAGB specifically in the context reflux prevention and resolution would be of significant value. In summary, while both RYGB and OAGB are effective bariatric procedures, this meta-analysis supports the superior reflux-related outcomes associated with RYGB. These findings are particularly relevant when selecting a surgical approach for patients with existing GERD or those undergoing revisional surgery. Careful preoperative evaluation and individualized risk assessment remain central to optimizing patient outcomes
Conclusions
The meta-analysis of the best available evidence demonstrated that OAGB is associated with inferior GERD outcomes compared to RYGB, with significantly higher rates of de novo GERD, greater symptomatic burden, and more frequent endoscopic oesophagitis. These findings raise concerns about the routine use of OAGB in patients with pre-existing GERD. Potential variation in diagnostic thresholds and reporting of GERD-related complications across studies may influence the observed outcome disparities. We recommend that OAGB be used with caution in patients with known GERD, and that surgical planning be individualised with thorough preoperative evaluation to guide the choice between OAGB and RYGB.
Compliance with Ethical Standards
Ethical Approval Considering the design of our study, ethical approval and consent were not required.
Conflict of Interest The authors declare no competing interests.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Angrisani L, Santonicola A, Iovino P, Ramos A, Shikora S, Kow L. Bariatric Surgery Survey 2018: Similarities and Disparities Among the 5 IFSO Chapters. Obes Surg. 2021; 31: 1937-1948.
- Maleckas A, Gudaityt? R, Petereit R, Venclauskas L, Veli?kien? D. Weight regain after gastric bypass: etiology and treatment options. Gland Surg. 2016; 5: 617-624.
- Ghanem OM, Ghazi R, Abdul Razzak F, Bazerbachi F, Ravi K, Khaitan L, et al. Turnkey algorithmic approach for the evaluation of gastroesophageal reflux disease after bariatric surgery. Gastroenterol Rep (Oxf). 2023; 11: goad028.
- Elzouki AN, Waheed MA, Suwileh S, Elzouki I, Swehli H, Alhitmi M, et al. Evolution of gastroesophageal reflux disease symptoms after bariatric surgery: A dose-response meta-analysis. Surg Open Sci. 2021; 7: 46-51.
- Vilallonga R, Sanchez-Cordero S, Umpiérrez Mayor N, Molina A, Cirera de Tudela A, Ruiz-Úcar E, Carrasco MA. GERD after Bariatric Surgery. Can We Expect Endoscopic Findings? Medicina (Kaunas). 2021; 57: 506.
- Ashrafi D, Osland E, Memon MA. Bariatric surgery and gastroesophageal reflux disease. Ann Transl Med. 2020; 8: S11.
- Felsenreich DM, Steinlechner K, Langer FB, Vock N, Eichelter J, Bichler C, et al. Outcome of Sleeve Gastrectomy Converted to Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass. Obes Surg. 2022; 32: 643-651.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339: b2700.
- Wells GA, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- Higgins JPT, Savovi? J, Page MJ, et al. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.1. Cochrane; 2020.
- Dayan D, Kanani F, Bendayan A, Nizri E, Lahat G, Abu-Abeid A. The Effect of Revisional One Anastomosis Gastric Bypass After Sleeve Gastrectomy on Gastroesophageal Reflux Disease, Compared with Revisional Roux-en-Y Gastric Bypass: Symptoms and Quality of Life Outcomes. Obes Surg. 2023; 33: 2125-2131.
- El Masry MAM, Abdul Rahman I, Elshal MFM, Abdul Moneim AM. Comparative study of midterm outcomes between Roux-en-Y gastric bypass (RYGB), diverted one-anastomosis gastric bypass (D-OAGB), and one anastomosis gastric bypass (OAGB). Langenbecks Arch Surg. 2024; 409: 340.
- Eskandaros MS, Abbass A, Zaid MH, Darwish AA. Laparoscopic one anastomosis gastric bypass versus laparoscopic Roux-en-Y gastric bypass effects on pre-existing mild-to-moderate gastroesophageal reflux disease in patients with obesity: a randomized controlled study. Obes Surg. 2021; 31: 4673-4681.
- Genco A, Castagneto-Gissey L, Gualtieri L, Lucchese M, Leuratti L, Soricelli E, et al. GORD and Barrett's oesophagus after bariatric procedures: multicentre prospective study. Br J Surg. 2021; 108: 1498-1505.
- Level L, Rojas A, Piñango S, Avariano Y. One anastomosis gastric bypass vs. Roux-en-Y gastric bypass: a 5-year follow-up prospective randomized trial. Langenbecks Arch Surg. 2021; 406: 171-179.
- Mustafa A, Rizkallah NNH, Samuel N, Balupuri S. Laparoscopic Roux-En-Y gastric bypass versus one anastomosis (loop) gastric bypass for obesity: A prospective comparative study of weight loss and complications. Ann Med Surg (Lond). 2020; 55: 143-147.
- Petrucciani N, Benois M, Aurello P, Boudrie H, VAN Haverbeke O, Barone SC, et al. Analysis of Factors Related to Gastroesophageal Reflux after Gastric Bypass at 10-Year Follow-up: A Retrospective Single-institutional Study. In Vivo. 2024; 38: 982-989.
- Poublon N, Chidi I, Bethlehem M, Kuipers E, Gadiot R, Emous M, et al. One anastomosis gastric bypass vs. Roux-en-Y gastric bypass, remedy for insufficient weight loss and weight regain after failed restrictive bariatric surgery. Obes Surg. 2020; 30: 3287-3294.
- Rheinwalt KP, Schipper S, Plamper A, Alizai PH, Trebicka J, Brol MJ, et al. Roux-en-Y Versus One Anastomosis Gastric Bypass as Redo-Operations Following Sleeve Gastrectomy: A Retrospective Study. World J Surg. 2022; 46: 855-864.
- Robert M, Poghosyan T, Maucort-Boulch D, Filippello A, Caiazzo R, Sterkers A, et al. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass at 5 years (YOMEGA): a prospective, open-label, non-inferiority, randomised extension study. Lancet Diabetes Endocrinol. 2024; 12: 267-276.
- Wilczy?ski M, Spychalski P, Proczko-Stepaniak M, Bigda J, Szyma?ski M, Dobrzycka M, et al. Comparison of the Long-term Outcomes of RYGB and OAGB as Conversion Procedures After Failed LSG - a Case-Control Study. J Gastrointest Surg. 2022; 26: 2255-2265.
- Hany M, Zidan A, Elmongui E, Torensma B. Revisional Roux-en-Y Gastric Bypass Versus Revisional One-Anastomosis Gastric Bypass After Failed Sleeve Gastrectomy: a Randomized Controlled Trial. Obes Surg. 2022; 32: 3491-3503.
- Chiappetta S, Stier C, Scheffel O, Squillante S, Weiner RA. Mini/One Anastomosis Gastric Bypass Versus Roux-en-Y Gastric Bypass as a Second Step Procedure After Sleeve Gastrectomy-a Retrospective Cohort Study. Obes Surg. 2019; 29: 819-827.
- Rayman S, Assaf D, Azran C, Sroka G, Assalia A, Beglaibter N, et al. Sleeve Gastrectomy Failure-Revision to Laparoscopic One-Anastomosis Gastric Bypass or Roux-n-Y Gastric Bypass: a Multicenter Study. Obes Surg. 2021; 31 : 2927-2934.
- Karagul S, Senol S, Karakose O. One anastomosis gastric bypass versus Roux-en-Y gastric bypass: a randomized prospective trial. Medicina (Kaunas). 2024; 60: 256.
- Singh B, Saikaustubh Y, Singla V, Kumar A, Ahuja V, Gupta Y, et al. One Anastomosis Gastric Bypass (OAGB) vs Roux en Y Gastric Bypass (RYGB) for Remission of T2DM in Patients with Morbid Obesity: a Randomized Controlled Trial. Obes Surg. 2023; 33: 1218-1227.
- Fahmy MH, Sarhan MD, Salman MA. Gastro-esophageal reflux disease after laparoscopic mini-gastric bypass and Roux-en-Y gastric bypass: is there a difference? Bariatr Surg Pract Patient Care. 2018; 13: 109-114.
- Lee WJ, Yu PJ, Wang W, Chen TC, Wei PL, Huang MT. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg. 2005; 242: 20-28.
- Delko T, Kraljevi? M, Lazaridis II, Köstler T, Jomard A, Taheri A, et al. Laparoscopic Roux-Y-gastric bypass versus laparoscopic one-anastomosis gastric bypass for obesity: clinical & metabolic results of a prospective randomized controlled trial. Surg Endosc. 2024; 38: 3875-3886.
- Abd El-Sadek MEG, Swelam AI, Abdallah HS. Short-term outcomes of laparoscopic Roux-en-Y gastric bypass versus laparoscopic one-anastomosis gastric bypass in super obese patients: randomized clinical trial. J Surg. 2021; 9: 209-215.
- Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN. Early Increases in Bile Acids Post Roux-en-Y Gastric Bypass are Driven by Insulin-Sensitizing, Secondary Bile Acids. J Clin Endocrinol Metab. 2015; 100: E1225-E1233.
- Benotti PN, Forse RA. The role of gastric surgery in the multidisciplinary management of severe obesity. Am J Surg. 1995; 169 :361-367.
- Arterburn D, Gupta A. Comparing the Outcomes of Sleeve Gastrectomy and Roux-en-Y Gastric Bypass for Severe Obesity. JAMA. 2018; 319: 235-237.
- Mickevicius A, Sufi P, Heath D. Factors predicting the occurrence of a gastrojejunal anastomosis leak following gastric bypass. Wideochir Inne Tech Maloinwazyjne. 2014; 9: 436-440.???????