ATP-Binding Cassette Cholesterol Transporter Family and Hyperlipidemia
- 1. Department of Endocrinology and Metabolism Disease, Sifa University Health Application and Research Center, Turkey
Abstract
Genetic characterisitics of the individuals lead to the continuation of normal functions of the body by realizing their effects on metabolic pathways. Aminoacid changes that occur on the structures of the genes effective on lipid metabolism (genetic polymorphisms) also change the functions performed by gene. As a result of this change, apolipoproteins in proteins related to lipoprotein metabolism often affect the changes in receptors, enzymes, or cofactors. Such changes occurring in connection with genetic changes are classified as primary disorders of the lipid mechanism. Changes occurring in ATP-Binding Cassette Cholesterol Transporter (ABCA1) gene may play an important role amongst these changes.
Citation
Karadeniz M (2013) ATP-Binding Cassette Cholesterol Transporter Family and Hyperlipidemia. J Endocrinol Diabetes Obes 1(2): 1011.
INTRODUCTION
Hyperlipidemia depends on concentration increase of plasma lipoproteins. One or more lipoprotein class can be accumulated in the blood as a result of increased synthesis or excessive blood release to the circulation or decreased cleareance or a defect in removal from the circulation. These changes in metabolic events are frequently connected to the changes in apolipoproteins, receptors, enzymes, or cofactors in proteins related to lipoprotein methabolism. These kinds of changes arising in connection with genetic changes are classified as primary lipid disorders. The changes happened in ATP-Binding Cassette Cholesterol Transporter (ABCA1) gene can play an important role amongst these changes. Firstly, ABCA1 gene was held responsible for the cause of Tangier disease. In this disease, a problem occurs in cholesterol transport associated with High-density cholesterol (HDL) between the tissue and the liver.
Mutations and alleles showing the defect in ABCA1 gene have also been reported in family hypoalfalipoproteinemia disease. Genetic problems in ABCA1 gene have also been reported in patients whose HDL- cholesterol is low without classical symptoms of Tangier disease.
Other cells, except for the cells in liver and steroidogenic tissues, cannot metabolize the cholesterol. Instead of this, these cells control novo cholesterol biosynthesis and cholesterol intake through low-density cholesterol (LDL) receptor. This mechanism is arranged in a manner that it will not allow excessive cholesterol load in cell membrane in many cell types. Some cells, especially macrophages, absorb cholesterol by endocytosis and phagocytosis, but they don’t have feedback control on cholesterol methabolism. These cells store excessive cholesterol in form of ester and secrete them when necessary (1-3).
HDL-cholesterol takes free cholesterol from other lipoproteins or cell membrane that has excessive cholesterol. These precursors in form of disc partially take free cholesterol. They are transformed from disc form to globular form by taking the cholesterol to their structure. The enzyme that esterified by taking free cholesterol in plasma is lecithin cholesterol acyl transferase (LCAT). Mature globular HDL (HDL3)- cholesterol increases its volume by also taking free cholesterol and forms HDL2- cholesterol. HDL2- cholesterol becomes very rich in ester. HDL- cholesterol that includes ApoE (HDL1) is found in lesser ratio but is metabolic active. Its HDL- cholesterol in presence of ApoE runs to LDL receptor. If HDL- cholesterol molecule does not include ApoE, it cannot interact with LDL receptor. Apo E is produced in liver, principally in macrophages. Apo E release can be stimulated HDL- cholesterol and Apo A1. However, Apo E gives positive contributions at receptor level during cholesterol intake of HDL- cholesterol from peripheries. While Apo E4 activity in Apo E genotype is decreased, the effect of Apo E2 is more increased (4-5).
HDL- cholesterol ensures the transfer of the lipids between lipoproteins and cells. It takes place in the center of the event that is known as reverse cholesterol transport. HDL- cholesterol takes cholesterol from the cells and transfers it to the liver for clearance or to the cells that need cholesterol. HDL3- cholesterol is transferred to HDL2- cholesterol and then transformed to HDL1- cholesterol. Cholesteryl ester transfer protein (CETP) molecule carries cholesterol ester to intermediate-density lipoprotein (IDL)- cholesterol and chylomicron residues. Thus, cholesterol is conveyed to the liver through VLDL-cholesterol and chylomicron residues. Also, triglyceride is carried to HDL2- cholesterol through CETP effect. CETP pathway is the principal way when carrying cholesterol from HDL to the liver. Hepatic lipase (HL) transforms HDL2-cholesterol to HDL3-cholesterol by hydrolyzing HDL2 triglyceride. Thus, HDL2-3 cycle continues. A sum of HDL is also taken by liver and is destroyed (6). High-density lipoprotein (HDL) carries 1/3 of the cholesterol in human plasma and is associated with the transport of excessive cholesterol from the cells. ?t is a heterogenous and multi-functioned molecule that regulates the transport of lipophilic molecule and lipids between HDL lipoprotein and the tissues. One of its most important functions is to mediate the transport of the cholesterol from peripherical tissues to the liver for discharge through bile (7-8).
After having combined with Apo-A1 or HDL-cholesterol in form of free cholesterol esters through LCAT enzyme, it is carried to the liver or inside of the macrophages directly or indirectly. HDL-cholesterol usually uses direct way for releasing HDL cholesterol (80%). However, indirect way is used in a frequency of 20%.
Direct way, Apo A-I is snythesized by liver an bowel.
- Hepatocyte
-Takes cholesterol esters by interacting wiht ABCA-I on macrophage
- Contributes to phospolipid transfer protein (PLTP) HDL-2
(Newly formed HDL, pre-beta HDL).
All HDL molecule are not taken inside of the cell during selective intake. CE (cholesterol ester) intake is not realized in other scavenger receptor (SR) family member through binding to the receptor. After HDL is bound to scavenger receptor class B1 (SR-B1), an hydrophobic canal is formed for taking KE on the surface of the cell. CE is taken from this canal to the insie of the cell and thus HDL-cholesterol levels are preserved (9-10). However, in other way, Apo E is taken inside of the cholesterol cell together with HDL-cholesterol with the help of cubilin, and HDL-cholesterol is broke up and transformed to its aminoacids.
In indirect way, other enzyme pathways such as CETP, HL, and endothelial lipase give function. In indirect way, there is a cholesterol change from HDL-cholesterol through triglyceride from lipoproteins containing Apo B by CETP effect.
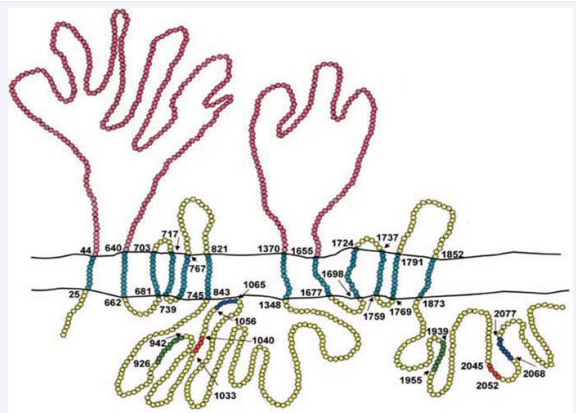
Macrophages, liver cells, and fibroblasts can re-secrete non lipid lipoproteins by loading lipid on them by taking HDL and chylomicron residuals inside the cell. This event is called as retroendocytosis and causes problem in this part in Tanger disease. Mutation happens in ATP binding cassette transporter 1 (ABC1) that ensures cholesterol output from the cell and as a result of this, HDL lipoprotei levels that are rich in lipid in the circulation are decreased. Mutation in both alleles forming ABC1 gene decreases HDL cholesterol and increases coronary artery disorder risk. To date, mutation formed in many regions of ABC1 gene has been reported (Figure 1).
Figure 1: Regions where reported mutations are formed on ABC 1 gene (11).
ABC1 is present in many cells (such as liver and bowel cells). This protein not only serces in cholesterol output from the cell but also takes an important role in formation of HDL from the bowel and the liver. Lipid-poor HDL precursors develop mature, lipid loaded, rounded HDL form with the help of other apoproteins by participation of phopholipids and formation of cholesterol esterified from cholesterol that is not esterified through LCAT from lipoproteins containing Apo-B. Small HDL3 molecules are intial form, and through LCAT esterification of the cholesterol and removal and addition of remnants from the surface of lipoproteins rich in triglyceride of other HDL3 molecules, HDL2 molecules are formed. Lipid and proteint content of HDL is cleaned from the circulation in two ways. The first way is formed by intake of lipids together with Apo E or Apo A1 through Sr (scavenger receptor)-B1. However, other way is indiract pathway related to CETP, HL, and endothelial lipase. HDL3 is formed as a result of intake from the lipids from HDL2 by SR-B1, CETP, and HL. The transformation of HDL3 to HDL2 is realized by PLTP, and at this time, ApoA-I without lipid is re-formed by pre-b1-Lpa-I These small apolipoproteins can easily exit to the outside of the vessel and contribute to HDL formation again or serve as cellular lipid receptors. These small particules in kidneys are discharged from plasma as fitler. Receive of Apo A1 from proximal tubule is realized through cubilin.
ATP-BINDING CASSETTE (ABC) FAMILY
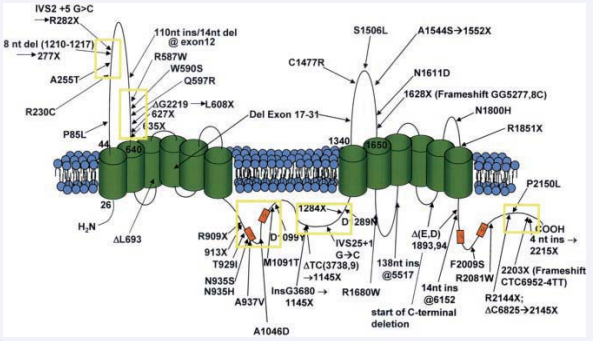
Luciani and collagues were defined ABC transporter family for the first time in 1994. ATP-binding cassette (ABC) genes encode a large family of transmembrane proteins. These proteins bind ATP in order to control the transition of different molecules from cell membranes (12). These proteins contain nucleotide binding flexions/loops (NBFs) depending on the organization and sequence of the region binding ATP. NBFs loops contain characteristic motifs (Walker A and B), these diverge by 90- 102 aminoacids and are bind to all ATP binding proteins. ABC genes carry C motif mark additionally and this is found at upper parts of Walker B. Functionally it contains two NBFs and two transmembrane proteins (TM). Transmembrane protein indexes are specified for 6-11 membrane cavity alfa helical structure (Figure 2).
Figure 2: Regions where reported mutations are formed on ABC 1 gene (11).
NBFs part is localized in cytoplasm and provides energy flow for membrane substrat transfer. ABC pumps are mostly single sided. They play role in intake of components (sugar, vitamins, and metal ions) that cannot pass into the cell through diffusion. Many ABC genes in eukaryotes are associated with substance transmit to the outside of the cell or the inside of cytoplasm from organelles [endoplasmic reticulum (ER), mitochondria, and peroxisome] (13-14).
Eukaryotic ABC genes include two pieces of TM and two pieces of NBF or half of them. ABC genes are largely found in eukaryote genoms and continue their functions beginning from early steps of the eukaryote development. This gene family is structurally divided into two sub groups (semi and full transporters). All human and rat ABC genes are developed by ABC gene researchers and are classified as standard by Human www.gene.ucl.ac.uk/nomenclature/genefamily/abc.html site. All ABC genes present in human are shown at this site. Liver X receptors (LXRs) are nuclear receptors obtained by cDNA in liver cell. They cause the formation of different transcriptional proteins when they are bound to the target (15). Later on, they are mentioned as “oxysterol receptors”. By behaving as a sensor in excess cholesterol formed in the body;
1) They inhibit cholesterol absorption from the bowel
2) They stimulate cholesterol output to HDL apolipoprotein through ABCA1 and ABCG1
3) They activate the transformation of the cholesterol in liver to bile acid
4) They activate the discharge of bile acids and biliary cholesterol
ABCA (ABC1)
Human contains 12 pieces of full transporter as ABCA sub-type. These are divided 2 subgroups based on intron structure and phylogenetic analysis. The first group contains 7 genes in 6 different chromosomes (ABCA1, ABCA2, ABCA3, ABCA4, ABCA7, ABCA12, ABCA13). On the other hand, the second group includes 5 genes aggregated on 17q24 chromosome ( ABCA5, ABCA6, ABCA8, ABCA9, ABCA10). ABCA subgroup represents a large group in 2100 aminoacid length of ABC genes. Second member of this family ABCA 1 and ABCA4 (ABCR) proteins have largely took place in studies. ABCA1 protein takes charge in HDL biosynthesis and cholesterol transport. ABCA4 protein is responsible for the transport of Vitamin A molecules at outer segments of rod photoreceptor cells that are an important step on visual cycle (11). ABCA1 gene is localized at 9q3 and 4 in human and rat genoms (16). ABCA1 gene is held responsible for the cause of Tangier disease. A problem happens in cholesterol transport depending on HDL between the tissue and the liver (9-10, 17-19). Mutations and alleles showing disorders in ABCA1 gene have also been reported for familial hypoalphalipoproteinemia disease (20). Genetic problems in ABCA1 gene have also been reported in patients with low HDl without classical symptoms of Tangier disease (21).
ABCA1 is responsinle for the transfer of these molecules to phospholipid and cholesterol receptors outside the cell. Lipid content of the membrane depends on ABCA1 and lateral wall is affected by plasticity and viscocity of membrane proteins and lipids. ABCA1 also takes charge effectively in the absorption of apoptotic materials (22-29).
The expression of ABCA1 is induced by sterol, nuclear hormon receptors, oxysterol receptors (LXR), bile acid receptors (FXR), and retinoic X heterodimers (30). It includes multple binding regions for binding transcription factors that are effective in lipid metabolism of promotor chain region (32-34).
In vitro liver X receptor agonists also inhibit prostate and breast cancer cell increase. LXR agonists causes p27 (kip) accumulation and G1 cycle insufficiency by decreasing Skp2 expression. At the same time, they hinder tumor progression by increasing ABCA1 expression in androgen dependent prostate tumors differently from androgen independent tumors. Phytosterols have been lately shown as LXR agonist as vegetal equivalent of mammal cholesterol. Betaacytosterol and campesterol (most frequently found two phytosterols) inhibit breast and prostate cancer increase. Anti-cancer activity of phytosterols may be related with the stimulation of LXR signal pathway (34).
Impairments in rat ABCA1 gene are associated with low HDL levels and accumulation of the cholesterol in tissues (35, 36). There is a defect in cholesterol transport from golgi apparatus to plasma membrane in analyses of ABCA1 _/_ performed in rats (35). On the other hand, cholesterol secretion to the inside of the bile in these rats are completely normal (37).
The event of cholesterol transport from artery wall or other tissues is known as “reverse cholesterol transport”. Recently, apolipoprotein A-I (apoA-I, the main protein structure of HDL) and initial lipidation of other apolipoproteins are ATP-binding cassette transporter A1 (ABCA1) system that is membrane lipid transporter and this system is speed limiting step in HDL fragment formation. Initial lipidation of HDL apolipoproteins affected by ABCA1 and vesicular transport system in many cells have not been understood yet and carry potential therapeutic importance. Niemann-Pick type C1 protein (NPC1) that is another protein and responsible for intracellular lipid transfer mutated in most of patients with niemann-Pick type C disease that is fatal neurodegenerative disorder. Mechanism of action of this protein could not be understood yet, but it is associated with the transport of the cholesterol from late endosome and lysosomes to other cell compartments. It is found in recent studies that there was a problem in lipid transport to ApoA-1 and regulation of ABCA1. At the same study, it is shown that there was an interaction between NPC1 and ABCA1 in cells with NPC disorder and that these two molecules realized arrangement of cholesterol transport together in the same study (38). Macrophage cells also express ABCG1 additionally to BCA1 (39-40). These enter into relation with ABCG1 lipid poor preß-HDL molecules, contrary to ABCA1. ABCG1 shows effect by decreasing cellular cholesterol transport, large spherical HDL2 and HDL3 molecules (41-42). For proper operation of reverse cholesterol transport, ABCA1 and ABCG1 need to operate synergistically (43). ABCA1 is also expressed in testis with the same elevation it has in the liver (44), this expression affects testicular cholesterol transport seperately from the circulation (45). Strong links in testis capillaries serve as a barrier in interstitium and leydig cell trannsition of plasma proteins (46).
ABCA2
Human ABCA2 protein is a molecule containing 2436 aminoacid and having the weight of 250 kDa (47). ABCA2 chromosome is localized at 9q and is associated with cholesterol efflux. Protein structure of ABCA2 resembles to that of ABCA1. Two symmetric proteins and a long cytoplasmic hydrophobic index between them take place in this structure. This long structure is embedded in cytoplasm (48). ABCA2 that is expressed in brain tissue have a different characteristic in its protein family with its characteristic. ABCA2 is localized in lysosomes and plays role in neuronal lipid transport (49). Promoter area of this gene is of importance as an important region in neural development phase, differentiation, and macrophage activation (48).
ABCA3
ABCA3 gene was cloned from human medullary cancer cell index in 1996. it resembles to ABCA1 and ABCA2 structurally. It is localized at chromosome 16p13, includes 1704-aminoacid, and its molecular weight is approximately 150kDa (50-51). ABCA3 is especially expressed in alveolar cells of type II in lungs. ABCA3 forms structures in shape of ring inside cytosol by using specific antibody in studies that are performed, and lamellar substances concentrate particularly in the membrane (52). Lamellar substances are the packages resembling dense liysosome that store pulmonary surfactant mixed with phospholipid and protein. These decrease the pressure in air-liquid contact point. Surfactant secretion plays an important role in orientation of lungs from liquid environment to air environment in birth.
ABCA5
ABCA5 protein shows maximal 42% of similarity to protein structures forming 17q24 chromosome. Therefore, it has an important characteristic among other members (53). Human ABCA5 protein carries 1642 aminoacids and has a weight of approximately 183 kDa. Recently, ABCA5 mRNA expression has been shown in Leydig cells where the testosterone is produced. Apart from that, it has been shown that most of these patinents die when they reach at their adulthood as a result of diseases involving lysosomal dysfunction of the tissues expressing ABCA5 (54).
ABCA6
Human ABCA6 protein contains 1617 aminoacid and carries a fully classical ABC transpoerter structure with the weight of 160 kDa (55). In humans, ABCA6 is expressed in different tissues (liver, lungs, heart, brain, and ovaries). It is shown that it is up-regulated during counter response to cholesterol concentration and macrophage differentiation (56). Therefore, it is thought that this protein plays an important role in macrophage lipid transport of this protein.
ABCA8
Human ABCA8 protein contains 1581 aminoacids and has a complete ABC transporter structure. It is thought that it is responsible for drug transport dependent on ATPaz and it has a substrate affinity very close to multidrug resistance-associated protein 2 (MRP2; ABCC2) (57-58). ABCA8 is expressed in differen mRNA tissues (liver, heart, muscle).
The ABCA1 has become a new therapeutic target for developing drugs designed for clearing cholesterol from arterial macrophages and preventing CVD. As example, prevention of oxidative damage to apolipoproteins in the vasculary wall could also be an important therapeutic focus for accelerating the ABCA1 pathway. Some factors that are elevated in individuals with MetS and diabetes, including fatty acids and glycoxidation end products, destabilize ABCA1 in experimental studies, increasing the possibility that damaged ABCA1 contributes to the rised CVD (59). Ibrolipim is a lipoprotein lipase (LPL) activator (60). In previously studies that ibrolipim increases lipoprotein lipase (LPL) messenger RNA in different tissues and LPL activity in post-heparin plasma, resulting in a reduction in triglyceride levels and rise of HDL-cholesterol. Some researchers showed that increasing LPL activity in skeletal muscle results in decreased fat accumulation, and long-term administration of ibrolipim protects against the development of atherosclerosis as experimental (61-62). ABCA1-activating drugs have the potential to mobilize cholesterol from macrophages of atherosclerotic lesions, making them powerful agents for preventing and reversing cardiovascular disease.
REFERENCES
3. Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev. 2003; 83: 1069-1112.
6. Ginsberg HN. Lipoprotein physiology. Endocrinol Metab Clin North Am. 1998; 27: 503-519.
20. Tall AR. Plasma cholesteryl ester transfer protein. J Lipid Res. 1993; 34: 1255-1274.
21. Steinberg D. A docking receptor for HDL cholesterol esters. Science. 1996; 271: 460-461.
28. Young SG, Fielding CJ. The ABCs of cholesterol efflux. Nat Genet. 1999; 22: 316-318.