Clinical Practice: New Perspective of Overweight and Obesity Causation
- 1. Department of Biological Sciences Microbiology PhD Program, Alabama State University, USA
- 2. Department of Internal Medicine, Jackson Hospital Montgomery, USA
- 3. Department of Pediatric Gastroenterology, Jackson Hospital Montgomery, USA
Abstract
Clinical Practice: New Perspective of Overweight and Obesity Causation.
Background: Within the United States ≈80.0% of the adult population is diagnosed with at least one non-communicable disease or disorder (NCD). Among these diseases, obesity is the leading cause of preventable death worldwide posing a major threat to human health and individual state-level health care systems.
Significance: Presently, there is an immediate need for a clinically revitalized perspective in overweight and obesity causation based upon several factors including; (i) the fact that the pathophysiology of theses conditions includes multiple sources of inflammation arising from metabolic, adipose tissue and gut microbiota influences, (ii) that gut microbiota can influence human obesity genes and, (iii) that consumption of highly processed food directly affects the structure and functionally of the gut microbiome leading to development of many NCDs.
Aim: Therefore, it is our aim here to provide physicians with a revitalized perspective of overweight and obesity causation in terms of the evolution of human diet and the impact is has upon the functionality of the gut microbiota.
Conclusion: Here we presented information that serves to assist health officials and physicians in redefining their clinical approach to the prevention and treatment of overweight and obesity as they are also considered to be as disorders associated with the overall health of the gut microbiota. A key initiative to bring awareness to the vital role of gut microbiota in human health, disease prevention and treatment and most importantly, to helping reduce the social stigmas associated with obesity and overweight.
Keywords
• Westernized diet
• Clinical practice
• Gut microbiome
• Obesity prevention
• Dysbiosis
Citation
Davis SC, Dosunmu-Ogunbi ABO, Dosunmu-Ogunbi SO, Barrow SD, Robertson BK (2016) Clinical Practice: New Perspective of Overweight and Obesity Causation. J Endocrinol Diabetes Obes 4(1): 1082.
ABBREVIATIONS
CD: Non-Communicable Disease or Disorder; BMI: Body Mass Index; Scfas: Short-Chain Fatty Acid; Mcfas; Medium-Chain Fatty Acid; Lcfas: Long Chain Fatty Acid; LPS: Lipopolysaccharides
INTRODUCTION
Within the United States ≈80.0% of the adult population is diagnosed with at least one non-communicable disease or disorder (NCD) such as obesity, diabetes, depression, cardiovascular disease, an intestinal disorder and or some type of cancer [1]. Among these diseases obesity is the leading cause of preventable death worldwide and within the United States, posing a major threat to human health and cause of social concerns as it associated with exponentially increasing morbidity along with direct and indirect socioeconomic costs [2-6]. Curtailing increasing rates is vital to the solvency of health care systems considering attributable costs are projected to reach $861-957 billion by the year 2030 [7,8]. Alternately, by reducing body mass index (BMI) of adults residents ≥ 5% by the same year, almost every state in the U.S. would save >$80 million (± 6.5 - 7.8 %) annually [9].
Besides genetic influence and or other medical reason, the causation of overweight and obesity have historically been and still are, associated with an increased intake of calories which exceeds the physical expenditures of the individual. However over the past decade, it has only been through use of scientific biotechnologies such as DNA sequencing platforms, that researchers now understand the causation from a much deeper, molecular level. Essentially, the causation of many NCD’s are directly associated with changes within the species of gut microbiota (residential bacteria populations of the large intestines), resulting in a disorder known as gut microbiome dysbiosis. It is also widely accepted among researchers that diet type is one of the most significant factors, besides antibiotic usage driving the composition and overall health of the gut microbiota [10-12]. While seemingly a mundane factor of causation, diet type is of even greater concern for physicians and patients in present day. Especially considering gut microbiota health as well as the fact that modern humans have an over abundant access to energy dense, nutrient poor food products specifically designed to induce repetitive eating behaviors [13,14].
Ultimately, overweight and obesity are multifaceted diseases associated foremost with the health of the gut microbiota but also with other deeply interwoven biological, environmental and social factors [15,16]. Adding to the complexity, information gleaned from studies using DNA sequencing biotechnologies have collectively illustrated that there is an immediate need for redefining the root ideal of causation of the two conditions. This need is realized as both are fundamentally found to be evolutionary based disorders intimately linked to the functionality of the gut microbiota. Redefining the root cause is also important as those presently accepted medically and socially reinforce social stigmas and severely inhibit prevention and treatment efforts within clinical practice [17,18]. It is our aim here, to provide physicians and public health officials with a revitalized perspective of overweight and obesity causation in terms of the evolution of human diet and the impact is has upon the functionality of the gut microbiota. It is our hope such information will assist state health officials and physicians in redefining their clinical approach to prevention and treatment of overweight and obesity as these conditions are now associated with disorders occurring within the microbiota populations.
Evolution of Human Diet & Obesity
Investigations into the diet of prehistoric ancestors provide evidence they consumed a raw, plant based diet compared to the primarily high animal protein diet consumed by many modern humans [19]. Determined through observations of jaw and specific tooth structures, a morphology defined as “taurodontism” was present among prehistoric human species, Homo gautengensis and Homo habilis (a known scavenger). The wide, flattened tooth morphology was reported to be suitable for masticating the fibrous plant material, whole nuts and grains constituting the early diet. In modern day however, evidence of taurodontism is present in diminishing degrees [20,21]. While sustainable growing and rapid food production are major global needs, processing methodologies such as grinding, grating, soaking, leaching, bleaching, peeling, drying, heat and antimicrobial treatments directly impact the physical, chemical and nutritional components of natural foods. The specific nutritional properties affected include the micro and macronutrient densities, fatty acid composition, glycemic load, sodium-potassium ratio as well as the overall natural fiber content [22]. Westernized diet type, which is growing in popularity within the United States is one based the consumption of manufactured or processed foods which are high in chemical additives, refined sugar, fats, fatty animal meats, salt and foods low in natural plant fiber [23-25].
Since the dawn of human existence, an array of microbes found the body to be a viable environmental niche with hundreds of species living on and within humans. Of all the residential microbes, the gut microbiota play such a vital role in human heath they are considered among researchers to be a living organ possessing their own unique anatomy and pathophysiology [26- 28]. Their contributions to human health have evolved to include a vast array of biological functions with the fundamental role still being that of a dietary energy extractor. Within the process of digestion the intestinal gut microbiota are needed to assist in the breakdown (fermentation) and absorption of nutrient molecules from resistant plant fibers, non-digestible plant cellulose, lipids, polysaccharides and resistant starches from processed foods that human enzymes cannot otherwise digest. Gut microbiota byproducts are also needed in the production of certain vitamins, hormones and energy molecules in the form of short-chain fatty acids utilized by the population as well as human cells throughout the body [29-36]. Additional evidence has shown the gut microbiota to be involved in several physiological processes associated with eating behaviors including the timing of dietary intake, satiety signaling, inducing food cravings and the regulation of systemic levels of fats and insulin [37-40].
However, because the population is a collection of living organisms the gut microbiota remain highly susceptible in utero, during infancy, throughout child and adulthood to factors such as antibiotics, exposures to other humans, human disease, medical interventions, natural environmental exposures and most importantly, the diet of the individual over the course of their life time [41,42]. Dietary investigations into the specific diets of carnivores, omnivores and herbivores reveled that both gut microbiota composition and the functionality of the gut microbiome (the collection of specific gut bacteria, their genes and byproducts), are adapted to the primary diet of the animal (human) host [43-45]. Therefore, despite promotion within the United States of a high protein diet as being health wise, there significant scientific evidence suggesting health care providers should refrain from endorsing such diet until more sound evidence of the risk-benefit ratio and impact upon gut microbiota are better determined [46]. Ultimately, it is within the role of the gut microbiota as an energy extractor and in their reaction to the drastic shifts in modern human diet from the primitive raw diet, the new perspective regarding the causation of overweight and obesity is realized.
Comparing Obesity Related Inflammation
Along with the vast array of other complex biological occurrences contributing to overweight and obesity causation, it is reported that (≈40-70%) of the diagnoses are attributed to genetic inheritability with melanocortin 4 receptor (MC4R), pro-opiomelanocortin (POMC), brain-derived neurotrophic factor (BDNF), leptin (LEP) and the leptin receptor (LEPR) genes encoding for the disease [47]. Albeit, considering the fact that consumption of a Westernized dietary regime is rapidly increasing, researchers now approach obesity studies using a ‘dietary-induced obesity model’ representative the energy dense, nutrient poor foods constituting the diet type. Combining such model with gut microbiota DNA sequencing platforms, scientists have found that the microbiota also significantly contribute to overweight and obesity causation through the induction of an inflammatory cascade and systemic reaction much different from the metabolically related inflammation historically associated these conditions [50,51]. Fundamentally, individuals possessing excess weight are in a constant state of chronic, low-grade systemic inflammation occurring within visceral and peripheral adipose tissues. Because of the chronic low-grade inflammation, these individuals are also at higher risk for development of many concomitant metabolic diseases and insulin resistance [48,49].
Metabolic Inflammation: Within humans, adipose tissue exists as either brown or white tissue types, with brown adipose primarily associated with in vivo thermogenic regulation. While brown adipose is also know to affect metabolism, white adipose serves as the site of energy storage and is directly involved in obesity related inflammatory cascades. Specifically, subcutaneous visceral white adipose deposits as well as those around internal organs including the heart and liver are the primary tissues associated with classic obesity related pathologies more so than peripheral subcutaneous adipose. As shown in (Figure 1),
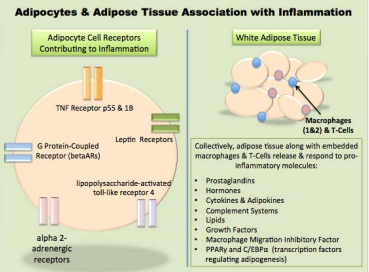
Figure 1 Factors of Adipocytes and White Adipose Tissue Contributing to Chronic Low-Grade Inflammation. As shown in the schematic, adipose tissue is a dynamic endocrine based organ intimately tied to chronic, low-grade inflammation as the excess dietary energy is stored within the cells. It is well understood that many biological and environmental factors can induce an inflammatory response within an adipocyte, which can in turn initiate an immune based inflammatory response through release of cytokine and hormone cascades. Additionally however, researchers now understand that the gut microbiota have propensity to influence the storage and release of energy within adipose and ultimate development of overweight or obesity through a condition known as gut microbiome dysbiosis.
white adipose is a complex metabolically active endocrine organ that directly influences energy harvest, storage, eating behaviors and can induce inflammatory cascades through production of various hormones, proteins, enzymes and adipocytokines [52].
Dietary fats vary in the length of their fatty acid chains and each are metabolized and or stored differently within the body. Among others, essential fatty acids include short-chain triglycerides; (SCFAs; 16 carbon fatty acids). In vivo, both SCFAs and MCFAs are rapidly absorbed directly into systemic circulation through the hepatic portal vein and transported to the liver for oxidation to be used as source of immediate energy. It has been suggested that increased dietary intake and metabolism of MCFAs may increase energy expenditure and decrease the deposition of lipids adipose tissue that results in faster satiety, contributing to weight loss. Long-chain triglycerides (12 carbons) however, are transported via chylomicrons into the lymphatic system allowing for an extensive uptake into adipocytes. It is the deposition of LCFAs as triglycerides into the adipocytes and other organ tissues is cornerstone to the classic metabolic inflammation associated with overweight and obesity [53]. Researchers also report that SCFAs have the propensity to induce overweight and obesity as the availability of intestinal energy is increased through consumption of modern day energy-dense food products; findings supported through evidence of obese individuals having higher fecal SCFAs than lean persons [54-57]. While the exact pathogenesis of metabolic inflammation is still be elucidated, overweight and obesity fundamentally manifest as multi-potent mesenchymal stem cells differentiate into mature adipocytes as the excess dietary energy is stored as lipid droplets within the cells [58].
Gut Microbiome Inflammation: Within the past decade researchers have come to understand that the gut microbiota can also induce a chronic, low-inflammatory reaction known as gut microbiome endotoxemia resulting in a condition referred to as adiposity (e.g., obesity) [59]. The root cause of gut microbiome endotoxemia and adiposity is associated with gut microbiome dysbiosis whereby the predominate phyla of gut microbiota populations shifts in the ratio of Bacteriodetes compared to Firmicutes and has an overall lower species diversity within the gut microbiota. Such changes directly influence the overall functionality and energy extracting capabilities of the gut microbiota and are also directly associated with the causation of many (NCDs) including obesity and type 2 diabetes [60- 64]. As summarized in (Figure 2),
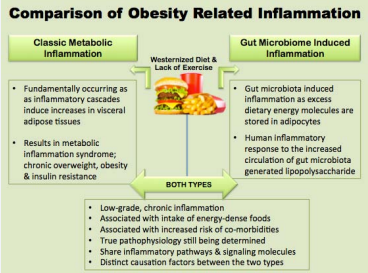
Figure 2 A Comparison of Overweight and Obesity Related Inflammation. Despite the fact the true pathology of metabolic inflammation and gut microbiome induced inflammation are still being elucidated, it is well known that both are associated with the increased consumption of a Westernized dietary regime, coupled with lack of physical activity. Additionally, while the fundamental causes of the types of inflammation arise from different sources (e.g., gut microbiome compared to adipose tissue), both types significantly contribute to and sustain the chronic low-grade inflammation associated with overweight and obesity.
compared to metabolic inflammation, the inflammation associated with gut microbiome endotoxemia is essentially a systemic immune reaction occurring in response to the increased circulation of gram negative microbial endotoxin, lipopolysaccharides (LPS). The increased levels within the intestinal lumen cause a thinning of the intestinal mucosal lining allowing the flow of (LPS) which is toxic to humans, directly into the circulatory system. The endotoxin activates an inflammatory cascade within adipocytes through stimulation of the lipopolysaccharide-activated toll-like receptor 4, which then induces nuclear factor NF-κB signal transduction pathways. Ultimately resulting in an inflammatory cascade (e.g., adiposity) following pathways similar to those of classic metabolic and immune inflammatory response. In late phase gut microbiome dysbiosis presents clinically as chronic overweight and eventually obesity [65-67].
Murine studies provide the foundation of understanding the associated effects of a high fat diet upon the gut microbiota. Researchers of these studies reported such diets induce macrophage infiltration, inflammation within adipose tissue, increase in circulating pro-inflammatory cytokines and induce Toll-like receptor 4 (TLR4), iNOS, COX-2 and colonic NF-κB [68 Kim 2012]. Gordon and colleagues are attributed with reporting the first definitive evidence illustrating the role of the gut microbiome in regulation of host energy homeostasis. Their investigations ultimately demonstrated that despite the germ-free mice being fed diets comprised of 29% higher caloric value than conventionally raised study mice, the germ-free mice had 40% less total body fat [69,70]. Additionally, other researchers report that gut microbiota can synthesize LCFAs by mechanisms similar to those found in adipose tissue as fatty acids are abundant in the lipids of many gut microbiota species [71,72].
New Perspective in Obesity Causation
The underlying principle of the revitalized perspective in overweight and obesity causation is based upon several factors including; (i) the fact that the pathophysiology of the conditions includes multiple sources of inflammation arising from metabolic, adipose tissue and gut microbiota influences, (ii) that gut microbiota can influence human obesity genes and, (iii) that consumption of highly processed food directly affects the structure and functionally of the gut microbiome leading to development of many NCDs. Now ‘normal weight obesity,’ a phenomenon whereby there is an increased percentage of total body adipose tissue and high degree of metabolic dysregulation within a normal weight individual. A condition presenting unique challenges for health care providers as the occurrence is result of gut microbiome dysbiosis; in early phase is asymptotic and undetectable without a DNA screening of patients stool sample [73].
Controlled feeding experiments have demonstrated dietary induced alterations within the composition of the gut microbiota can occur within 24 hours. Although, considering fad diets and diet therapy as part of treatment plans for overweight and obese patients these rapid changes are potentially short-term depending upon the degree of variation in diet, duration of the change as well as the early structure of the gut microbiota during the childhood. Important factors as the resilient gut microbiota often regress back to the malnourished, obese or disrupted state prior to dietary interventions [74-77]. Albeit, many studies have suggested that future clinical prevention and or treatment of many NCDs such as overweight and obesity will be aimed at restoring the gut microbiome dysbiosis through intensive collaborative therapies including dietary and behavioral modifications, increased physical activity, customized pre and pro-biotic therapies and or in extreme cases fecal transplant therapy [78,79].
CONCLUSION
Here we have presented here rationale for a revitalized perspective in overweight and obesity causation and in how these conditions are approached from social, public health and medical perspectives [80-82]. A key initiative to bring awareness to the vital role of gut microbiota in human health, reducing associated social stigmas, to increasing social movements geared towards sustaining decreases in obesity rates and to redefine clinical approach to prevention and treatment of overweight and obesity [83].
ACKNOWLEDGEMENTS
Administrative and Medical Staff of Dr. Adedoyin D. Ogunbi, Jackson Hospital. Administrative and Medical Staff of Dr. Sesi D. Ogunbi, Jackson Hospital. Sheree J. Finley, Department of Biological Sciences & Physical Science Department, Forensic Program, Alabama State University. Microbiology PhD Program Staff and Students, Department of Biological Sciences Alabama State University
Conflict of Interest Statement & Study Funding
The authors have no competing interests to declare. Present study was funded through National Science Foundation Grant: Number-1432991.
REFERENCES
2. Centers for Disease Control. Obesity Causes | Adult | Obesity | DNPAO | CDC’. Cdc.Gov. 2015.
4. Centers for Disease Control. ‘Obesity Causes | Adult | Obesity | DNPAO | CDC’. Cdc.Gov.
7. American Heart Association. ‘Obesity Statistical Fact Sheet 2013 Update.’. Heart.Org. 2013.
13. Müller, Thaddeus. Contributions from European Symbolic Interactionists.
19. Breasted, James Henry. ‘Ancient Times. A History Of The Early World’. The Art World. 1918; 3: 337.
20. Breasted, James Henry. Ancient Records Of Egypt. [Whitefish, MT]: Kessinger Pub. 2003.
51. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011; 29: 415-445.
60. Xu Z, Knight R. Dietary effects on human gut microbiome diversity. Br J Nutr. 2015; 113 Suppl: S1-5.









































































































































