Combination Therapy of Mitiglinide with Alpha-Glucosidase Inhibitor and or or DPP-4I Improves Platelet-Derived Microparticle and Adiponectin Levels in Patients with Type 2 Diabetes Mellitus
- 1. Department of Internal Medicine, Kansai Medical University, Japan
- 2. Division of Internal Medicine, Kohrigaoka Yukeikai Hospital, Japan
- 3. Division of Internal Medicine, Daiwa Hospital, Japan
- 4. Division of Internal Medicine, Meisei Memorial Hospital, Japan
- 5. Division of Internal Medicine, Saiseikai Izuo Hospital, Japan
Abstract
Background: Type 2 diabetes mellitus (T2DM) is an important risk factor for atherosclerotic cardiovascular disease. To investigate the effect of mitiglinide in combination with an alpha-glucosidase inhibitor (αGI) and/or DPP-4I on circulating levels of PDMPs, sCD40L, sE-selectin, sVCAM-1 and adiponectin in patients with T2DM.
Methods: This is a prospective cohort study. A total 79 patients who were selected from among those admitted to our hospital for the treatment of hypertension, hyperlipidemia, and diabetes between April 2012 and September 2014. Mitiglinide (30 mg/day) was administered for 3 months. Levels of IL-6, MCP-1, PDMP, sCD40L, RANTES, sE-selectin, sVCAM-1, and adiponectin were measured by ELISA at baseline and after 3 months of treatment.
Results: The levels of IL-6, PDMP, sCD40L, RANTES, sE-selectin and sVCAM-1 were higher in diabetic than in nondiabetic patients, while the adiponectin levels of diabetic patients were lower than those of nondiabetic patients. Mitiglinide therapy significantly decreased plasma PDMP, sCD40L, RANTES, sE-selectin and sVCAM-1 levels relative to baseline, and caused a significant increase in adiponectin compared with baseline. Furthermore, the modulation of biomarkers by mitiglinide therapy was significantly greater in the combination therapy group (mitiglinide with αGI and/or DPP-4I) than the mitiglinide monotherapy group.
Conclusion: Combination therapy of mitiglinide with αGI and/or DPP-4I has an adiponectin-dependent antiatherothrombotic effect that may be beneficial for the primary prevention of atherothrombosis in patients with T2DM.
Keywords
• Type 2 diabetes mellitus
• Mitiglinide
• α-GI
• DPP-4I
• Platelet activation markers
• Adiponectin
Citation
Nomura S, Omoto S, Taniura T, Okuda Y, Shouzu A (2016) Combination Therapy of Mitiglinide with Alpha-Glucosidase Inhibitor and/or DPP-4I Improves Platelet-Derived Microparticle and Adiponectin Levels in Patients with Type 2 Diabetes Mellitus. J Endocrinol Diabetes Obes 4(1): 1081.
ABBREVIATIONS
T2DM: Type 2 Diabetes Mellitus; PDMPs: Platelet-Derived Microparticles; DPP-4I: Dipeptidyl Peptidase-4 inhibitor; αGI: Alpha-Glucosidase Inhibitors; NO: Nitric Oxide; IL-6: Interleukin-6; MCP-1: Monocyte Chemoattractant Protein-1; RANTES: Regulated on Activation Normally T-cell Expressed and Secreted; sCD40L: Soluble CD40 ligand; sE-Selectin: Soluble E-Selectin; sVCAM-1: Soluble Vascular Cell Adhesion Molecule; GLP-1: Glucagon-Like Peptide 1.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is an important risk factor for atherosclerotic cardiovascular disease [1,2]. A repetitive postprandial rise in blood glucose is now considered a risk factor for the progression of atherosclerosis [3-6]. In addition, platelet activation and endothelial dysfunction are key features of atherosclerosis and contribute to the development of clinical cardiovascular disease [7,8]. Therefore, the mechanism by which platelet activation and endothelial dysfunction contribute to atherothrombosis is important in T2DM.
Platelet-derived microparticles (PDMPs) participate in normal haemostatic responses to vascular injury as they possess prothrombotic activity [9-11]. PDMPs are also released from platelets following physical stimulation under various conditions [9-13] and contribute to thrombin generation and thrombus formation by generating tissue factors [10,13]. Therefore, PDMPs can ultimately lead to vascular complications through their role in the blood coagulation system.
Adiponectin, the most abundant adipose tissue-specific protein, is exclusively expressed in and secreted by adipose tissue [14]. Plasma adiponectin concentrations have been shown to be decreased in obese individuals with type 2 diabetes and to be closely related to whole-body insulin sensitivity [15,16]. The protein occurs in abundance in the circulation and stimulates nitric oxide (NO) production in vascular endothelial cells which ameliorates endothelial cell function [17-19]. These observations suggest that the anti atherogenic properties of adiponectin may involve its NO-dependent anti platelet effects.
Mitiglinide is a short-acting insulinotropic sulfonylurea receptor ligand that has been shown to improve postprandial hyperglycemia by its glucose-lowering effect [20]. Mitiglinide also inhibits postprandial hypertriglyceridemia in Otsuka Long-Evans Tokushima Fatty rats, which exhibit insulin resistance and visceral fat accumulation and are considered as an aging diabetes model [21]. Furthermore, mitiglinide reduces the levels of circulating biomarkers of oxidative stress and inflammation caused by postprandial hyperglycemia [22]. However, the effects of mitiglinide in monotherapy or combination therapy on PDMPs and adiponectin in patients with T2DM are unclear. Therefore, this study aimed to investigate the effects of mitiglinide-related therapy on PDMPs and adiponectin in T2DM patients.
MATERIALS AND METHODS
Study subjects
The subjects included 38 nondiabetic and 41 diabetic patients (Table 1).
Table 1: Demographic and clinical characteristics of the diabetic patients and non-diabetic controls, Data are shown as the mean ± SD. N.S.; not significant P value, diabetic patients vs non-diabetic controls, BMI: body mass index; FBG: fasting blood glucose; HbA1c: hemoglobin A1c; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; ARB: angiotensin II receptor blocker.
| Non-diabetic controls | diabetic patients | p value | |
| No. of patients | 38 | 41 | N.S. |
| Gender(male/female) | 18/20 | 20/21 | N.S. |
| Age,y | 63±10 | 67±11 | N.S. |
| BMI,kg/mv² | 25.8±3.9 | 26.7±2.3 | N.S. |
| FBG,mg/dl | 100±23 | 177±29 | <0.01 |
| HbA1c,% | 5.1±1.1 | 7.9±0.6 | <0.01 |
| TC,mg/dl | 219±31 | 226±38 | N.S. |
| TG,mg/dl | 190±40 | 183±33 | N.S. |
| HDL-c,mg/dl | 48±14 | 51±7 | N.S. |
| LDL-C,mg/dl | 150±39 | 157±30 | N.S. |
| Complications,n(%) | |||
| Angina pectoris | 5(13.2) | 6(14.6) | N.S. |
| Heart faliure | 3(7.9) | 3(7.3) | N.S. |
| Cerebral infraction | 2(5.3) | 3(7.3) | N.S. |
| Medications,n(%) | |||
| Aspirin | 4(10.5) | 5(12.2) | N.S. |
| Statins | 11(28.9) | 9(22.0) | N.S. |
| ARBs | 15(39.5) | 13(31.7) | N.S. |
| Ca-antagonists | 10(26.3) | 10(24.4) | N.S. |
Between April 2012 and September 2014, patients were selected from among those admitted to our hospital for the treatment of hypertension, hyperlipidemia, and diabetes. The study protocol was approved by our Institutional Review Board (IRB) and written informed consent was obtained from each patient prior to starting the trial. A history (within 3 months prior to enrollment) of inflammatory disease, coronary artery disease, or cerebrovascular disease was not permitted. Clinically detectable renal dysfunction (serum creatinine ≥2.0 mg/dl), hepatic dysfunction (elevated transaminases), infection (fever or an elevated white blood cell count), or malignancy (detected by ultrasound or computed tomography) were also not permitted. Nine patients were taking aspirin because of old cerebral infarction or angina pectoris, while 28 patients were using angiotensin II receptor blockers (ARBs) and 20 patients were taking Ca-antagonists for hypertension (Table 1). There were also 20 patients taking statins for hyperlipidemia. The doses of prior drugs such as aspirin, statins, ARBs, and Ca-antagonists were not adjusted during the present study. Diabetic patients also included other anti-diabetic drugs such as 19 dipeptidyl peptidase-4 inhibitor (DPP-4I) and 21 alpha-glucosidase inhibitors (αGI).
Study design
Mitiglinide (30 mg/day) was administered for 3 months to randomly selected patients. There were no other changes to drug therapy during the treatment. Clinical and biochemical data were obtained before and after starting mitiglinide administration.
Measurement of platelet-derived microparticles
An enzyme-linked immunosorbent assay (ELISA) kit for the detection of platelet-derived microparticles (PDMP) [23-25] was obtained from Jimro Co., Ltd. (Tokyo, Japan). In brief, a blood sample was collected from a peripheral vein into a vacutainer containing EDTA-ACD (Nipro Co. Ltd., Japan) with a 21-gauge needle to minimize platelet activation. The sample was gently mixed by inverting the tube once or twice and then left at room temperature for 2-3 hr, followed by centrifugation at 8,000 g for 5 min at room temperature. Immediately after centrifugation, we collected 200 µl of the upper layer of supernatant from a 2-ml sample to avoid contamination and stored each sample at –40ºC until analysis. The results of the ELISA performed under the current experimental conditions were reproducible. PDMP were measured twice and the mean value was calculated. The kit employed two monoclonal antibodies directed against platelet glycoproteins CD42b and CD42a (glycoprotein Ib and IX). One U/ ml of PDMP was defined as 24,000 solubilized platelets/ml in this ELISA.
Measurement of interleukin-6, monocyte chemoattractant protein-1, regulated on activation normally T-cell expressed and secreted, soluble CD40 ligand, soluble E-selectin, soluble vascular cell adhesion molecule-1 and adiponectin: Fasting blood samples from patient and control subject peripheral veins were collected into vacutainers containing EDTA-ACD (NIPRO Co. Ltd., Osaka, Japan) using 21-gauge needles to minimize platelet activation. Samples were gently mixed by inverting the tubes once or twice and were then kept at room temperature for a maximum period of 2–3 h. Immediately after centrifugation at 8,000 g for 5 min, 200 μl of the upper layer supernatant from the 2 ml samples was collected to avoid contamination with platelets. The collected samples were stored at −40°C until analysis. Plasma interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), regulated on activation normally T-cell expressed and secreted (RANTES), soluble CD40 ligand (sCD40L), soluble E-selectin (sE-selectin) and soluble vascular cell adhesion molecule-1 (sVCAM-1) were measured using a monoclonal antibody-based ELISA kit purchased from Invitrogen International Inc. (Camarillo, CA, USA), while plasma adiponectin was measured with an Adiponectin ELISA kit purchased from Otsuka Pharmaceuticals Co. Ltd (Tokyo, Japan). Recombinant products and standard solutions provided with the commercial kits were used as positive controls in each assay. All kits were used in accordance with the manufacturer’s instructions.
Statistical analysis
Data were expressed as mean ± SD and were analyzed using multivariate regression analysis, as appropriate. Between-group comparisons were analyzed using the Newman–Keuls test and Scheffe’s test. The correlation between HbA1c concentration and continuous variables was assessed using multivariate linear regression analysis. The significance of differences among variables was determined by analysis of variance (ANOVA). P-values less than 0.05 were considered statistically significant. All analyses were performed using the StatFlex program (ver. 6).
RESULTS
Patient demographic and clinical characteristics were similar in the diabetic and nondiabetic groups, except for fasting blood glucose (FBG) and hemoglobin (Hb)A1c concentrations (Table 1).
Levels of IL-6, PDMPs, sCD40L, RANTES, sE-selectin and sVCAM-1 were higher in diabetic than in nondiabetic patients, while adiponectin levels were lower (Table 2).
Table 2: Plasma levels of cytokines, chemokine, soluble factors and adiponectin in the non-diabetic controls and diabetic patients, Data are shown as the mean ± SD. N.S.; not significant P value, diabetic patients vs non-diabetic controls, IL-6: interleukin-6; MCP-1: monocyte chemoattractant protein-1; PDMP: platelet-derived microparticle; sCD40L: soluble CD40 ligand; RANTES: regulated on activation normally T-cell expressed and secreted; sE-selectin: solubleE-selectin; sVCAM-1: soluble vascular cell adhesion molecule-1
| Non-diabetic controls | diabetic patients | p value | |
| IL-,pg/ml | 2.82±1.64 | 4.69±4.04 | <0.05 |
| M6P-1,pg/ml | 398±113 | 425±136 | N.S. |
| PDMP,u/ml | 15.2±5.1 | 23.1±8.9 | <0.01 |
| sCD40L,ng/ml | 21.3±0.93 | 2.76±0.71 | <0.05 |
| RANTES,pg/ml | 80±25 | 102±47 | <0.05 |
| sE-selection,ng/ml | 72±39 | 98±38 | <0.05 |
| sVCAm-1,ng/ml | 736±102 | 999±144 | <0.05 |
| Adiponectin,µg/ml | 4.53±0.97 | 2.3±0.92 | <0.01 |
Using univariate and multivariate regression analyses, we investigated the associations between HbA1c concentration and 14 other variables in diabetic patients (Table 3).
Table 3:
| Analysis | Univariate | Multivariate | ||
| β | p value | β | p value | |
| Age(years) | 0.3126 | 0.19234 | ||
| Sex(men) | -0.0561 | 0.37112 | ||
| BMI(kg/mv²) | 0.3866 | 0.01753 | 0.2814 | 0.21387 |
| TC(mg/dl) | -0.0927 | 0.24861 | ||
| HDL-C(mg/dl) | -0.1955 | 0.39814 | ||
| LDL-C(mg/dl) | 0.4633 | 0.02317* | 0.3129 | 0.06978 |
| IL-6(pg/ml) | 0.1527 | 0.021333 | ||
| MCP-1(pg/ml) | 0.4128 | 0.01559* | 0.3325 | 0.05671 |
| PDMP(u/ml) | 0.5639 | 0.00009* | 0.4013 | 0.00993* |
| sCD40L(ng/ml) | 0.4976 | 0.00039* | 0.3516 | 0.03211* |
| RANTES(pg/ml) | 0.4684 | 0.00142* | 0.3351 | 0.04124* |
| sE-selection(ng/ml) | 0.3421 | 0.00955* | 0.2638 | 0.09124 |
| sVCAM-1(ng/ml) | 0.4132 | 0.00114* | 0.3237 | 0.05126 |
| Adiponectin(µg/ml) | -0.6124 | <0.0001* | -0.4993 | 0.00367* |
Univariate analysis showed that body mass index (BMI), LDL, MCP-1, PDMPs, sCD40L, RANTES, sE-selectin and adiponectin were factors significantly associated with HbA1c, whereas PDMPs, sCD40L, RANTES and adiponectin were significantly correlated with HbA1c by multivariate analysis.
Administration of mitiglinide to diabetic patients for 3 months significantly reduced FBG and HbA1c levels (data not shown) and plasma concentrations of PDMPs, sCD40L, RANTES, sE-selectin and sVCAM-1, relative to baseline (Figure 1). Additionally, mitiglinide treatment significantly increased adiponectin concentrations after 3 months, relative to baseline (p<0.01) (Figure 1).
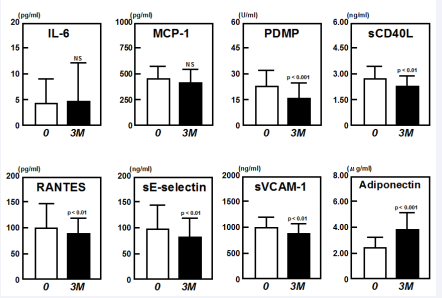
Figure 1
We divided the patients in the diabetes group into four subgroups, according to the mitiglinide combination treatment received. The plasma levels of PDMPs and adiponectin in the four groups (mitiglinide alone, n=8; mitiglinide with DPP-4I, n=12; mitiglinide with αGI, n=14; and mitiglinide with DPP-4I and αGI, n=7) are shown in Figure 2. All four groups exhibited significantly decreased PDMP levels after treatment. Using ANOVA analysis to compare the groups, significant changes in plasma PDMP levels were observed in the αGI-included groups (mitiglinide alone vs. mitiglinide with αGI, p < 0.05; mitiglinide alone vs. mitiglinide with DPP-4I and αGI, p<0.01). However, combination therapy of mitiglinide with αGI and/or DPP-4I exhibited a significant increase in adiponectin levels after treatment, whereas mitiglinide monotherapy did not. In addition, significant changes in adiponectin levels were observed in the combination therapy groups compared with monotherapy (mitiglinide alone vs. mitiglinide with DPP-4I, p < 0.05; mitiglinide alone vs. mitiglinide with αGI, p < 0.01; mitiglinide alone vs. mitiglinide with DPP-4I and αGI, p < 0.01).
Table 4: Changes in PDMP and adiponectin in response to treatment with mono- or combination therapy of mitiglinide in diabetic patients *: p values are for comparison with each baseline parameter (before vs. after treatment). **: ANOVA: analysis of variance (mitiglinide monotherapy vs. combination therapy).
| Before treatment | After treatment | p value | ANOVA* | |
| PDMP(u/ml) | ||||
| Mitiglinide(n=8) | 30.1±6.5 | 26.3±5.2 | <0.05 | |
| DPP4I-(n=12) | 28.9±6.1 | 23.9±5.7 | <0.05 | NS |
| +α-GI(n=14) | 32.5±7.3 | 19.6±6.1 | <0.01 | <0.05 |
| +DPP-4I& α-GI(n=7) | 29.2±5.9 | 14.1±6.3 | <0.001 | <0.01 |
| Adiponetin(µg/ml) | ||||
| Mitigidlinide(n=8) | 2.18±0.43 | 2.52±0.75 | NS | |
| +DPP-4I(n=12) | 2.29±0.37 | 3.44±0.37 | <0.05 | <0.05 |
| +α-GI(n=14) | 2.15±0.41 | 3.69±0.56 | <0.001 | <0.01 |
| +DPP-4I& α-GI(n=7) | 2.12±0.46 | 3.87±0.62 | <0.001 | <0.01 |
DISCUSSION
PDMPs play an important role in the clotting process, so increased levels are likely to cause hypercoagulability [9-11,26]. PDMP levels are significantly increased in diabetic patients and may participate in the development or progression of atherosclerosis in diabetes [27,28]. In this study, mitiglinide therapy significantly decreased plasma PDMP levels. Although no direct changes in platelet function were shown, mitiglinide therapy also decreased other platelet activation markers (sCD40L and RANTES) in diabetic patients. Postprandial hyperglycemia is thought to be an important cause of platelet activation in diabetes [29]. Oxidative stress is induced by postprandial hyperglycemia via various biochemical pathways, leading to the generation of superoxide, which reacts with NO to form peroxynitrite [30]. The resulting decrease in NO levels and activity may accelerate vascular inflammation and platelet activation by enhancing the expression of various cytokines and growth factors [31]. In this study, platelet and endothelial activation markers were elevated in diabetic patients compared with nondiabetic patients. Thus, our results indicate that postprandial hyperglycemia causes platelet activation and endothelial dysfunction and modulates the effect of mitiglinide on PDMPs.
Previous studies have indicated that treatment with mitiglinide significantly reduces BMI and waist circumference [32,33], and prevents endothelial dysfunction in T2DM [33]. Plasma levels of adiponectin, shown to be increased in this study, are decreased in obese individuals and T2DM as adiponectin is closely related to whole-body insulin sensitivity [14,34]. Adiponectin has been reported to suppress the attachment of monocytes to endothelial cells [31] and plays a role in protection against vascular injury; hypoadiponectinemia is therefore associated with endothelial dysfunction [35] and appears to cause platelet activation. NO regulates platelet activation and is decreased by hypoadiponectinemia because adiponectin stimulates NO production by vascular endothelial cells [18,19,36]. Thus, platelet activation occurs as a result of low NO concentrations in patients with hypoadiponectemia. Increased adiponectin caused by mitiglinide may therefore exert an antiplatelet effect by promoting NO production, and improvement of hypoadiponectinemia may result from decreased levels of PDMPs.
It has been shown that various posttranslational modifications, including glycosylation of lysine residues, are necessary for the multimerization of adiponectin to occur [37]. Such intracellular posttranslational processes may be affected by hyperglycemia, leading to functional impairment at the organ level in diabetic patients [38,39]. Therefore, the improvement in postprandial hyperglycemia by mitiglinide could alter the posttranslational modification of adiponectin. However, increased adiponectin caused by mitiglinide was not significant statistically in the patients treated with mitiglinide alone. In this study, we divided the diabetic patients into four subgroups by mitiglinide combination treatment. Significant changes in plasma PDMP levels were observed compared with baseline, most notably in the αGI-included groups. However, combination therapy of mitiglinide with αGI and/or DPP-4I significantly increased adiponectin levels, while mitiglinide monotherapy did not. Overall, the changes induced by αGI-included combination therapies were more considerable than in the other groups. These results suggest that combination therapy of mitiglinide with αGI and/or DPP-4I causes an adiponectin-dependent improvement in plasma levels of platelet and endothelial activation markers in diabetic patients. The benefits of combination therapy of mitiglinide with αGI and/or DPP-4I in T2DM have been previously reported [40-43]. Although these studies demonstrated enhanced glucose-lowering effects by combination therapies, effects on PDMP or adiponectin levels were not shown. The exact mechanism by which mitiglinide treatment with αGI and/or DPP-4I leads to an increase in circulating adiponectin levels remains unclear. We postulate the participation of the gut-derived incretin hormone, glucagon-like peptide 1 (GLP-1), for the mechanism underlying adiponectin elevation by αGI treatment [44]. The glucose-lowering and antiobesity effects of GLP-1-based therapies for T2DM have been greatly evaluated previously [45]. In addition, some studies showed that GLP-1 could promote adiponectin secretion [45,46]. We believe that the effect of mitiglinide αGI and/or DPP-4I on PDMP depends on adiponectin. Therefore, mitiglinide with αGI and/or DPP-4I could inhibit the progression of atherothrombosis by promoting adiponectin-dependent improvement of PDMPs and endothelial dysfunction. However, further studies are necessary to elucidate the effects of combination therapy itself on adiponectin production.
CONCLUSION
Mitiglinide treatment with αGI and/or DPP-4I increased circulating adiponectin levels in patients with T2DM and decreased platelet and endothelial cell activation markers. Mitiglinide in combination with αGI and/or DPP-4I may therefore be beneficial for the primary prevention of atherothrombosis in patients with T2DM. However, further studies are needed to confirm this hypothesis.
ACKNOWLEDGMENTS
This study was partly supported by a grant from the Japan Foundation of Neuropsychiatry and Hematology Research, a Research Grant for Advanced Medical Care from the Ministry of Health and Welfare of Japan, and a Grant (13670760 to S.N.) from the Ministry of Education, Science and Culture of Japan.
AUTHORS CONTRIBUTIONS
SN conceived and designed the research. SN, SO and AS performed the clinical study. SN and TT analyzed the data. SN and SO wrote the paper. MO and TT contributed equally to this paper.









































































































































