IL-35 Levels and Their Association with Thyroid Function Tests in Hashimoto
- 1. Department of Internal Medicine, University of Health Science, Ankara City Hospital, Ankara, Turkey
- 2. Department of Internal Medicine, Necmettin Erbakan University, Konya, Turkey
Abstract
Background: In this study, it was aimed to examine interleukin-35 (IL-35) levels and their association with thyroid function tests in Hashimoto’s thyroiditis (HT).
Methods: Included in the study were 128 individuals, between 18 and 65 years of age, who had been newly diagnosed with HT (euthyroid, subclinical hypothyroidism and overt hypothyroidism) and 38 health controls who had no known diseases or drug use.
Result: No significant difference was determined between the groups in terms of the IL-35 levels (P = 0.285), although in the in-group comparisons, the IL-35 levels were found to decrease progressively towards overt hypothyroidism. In the in-group comparisons, however, a statistically significant difference was determined between the control group and the overt hypothyroidism group (550.05 ± 411.50 vs. 369.80 ± 253.33; P = 0.046). When the patient groups were grouped according to their thyroid stimulating hormone values, a significant difference was determined between the groups with a threshold value of ≥6 uIU/ mL and those below it, in terms of the IL-35 levels (P = 0.043). When two groups were created, comprising those with a threshold value of ≥10 uIU/mL and those below it, it was observed that there was a more significant difference between the groups in terms of the IL-35 levels (P = 0.024). As a result of the correlation analysis performed by taking into account the controllable factors (smoking, diabetes mellitus, hypertension, and body mass index), a low-significant correlation was determined between the IL-35 levels and antithyroid peroxidase (P = 0.029).
Conclusion: In conclusion, in this study, it was determined that the IL-35 levels, an antiinflammatory cytokine involved in HT, decreased progressively from the euthyroid patient group towards the overt hypothyroidism group.
Keywords
- Hashimoto’s thyroiditis
- Antiinflammatory cytokine
- Autoimmunity
- IL-35
Citation
Inan O, Karakurt F (2021) IL-35 Levels and Their Association with Thyroid Function Tests in Hashimoto’s Thyroiditis. J Endocrinol Diabetes Obes 9(1): 1123.
INTRODUCTION
Hashimoto’s thyroiditis (HT) was originally defined by Japanese surgeon Dr. Hakaru in 1912, in 4 patients with chronic thyroid disease and enlarged thyroid glands [1]. HT is the most common global cause of hypothyroidism in iodinesufficient areas [2]. Furthermore, it is the most common cause of hypothyroidism in adults, with a recently increasing incidence [3,4]. HT is an autoimmune thyroid disease that is characterized by diffuse lymphocytic infiltration of the thyroid gland, which is clearly revealed by the presence of circulating thyroid autoantibodies and clinical or immunological co-existence with other autoimmune diseases [5,6]. This disease is also called chronic autoimmune thyroiditis [7].
Cellular and humoral immunity together play a role in the pathogenesis of HT and the activation of CD4 (+) T lymphocytes specific to thyroid antigens is thought to be the first step in its pathogenesis [8]. There is a cell-cell interaction and cytokine control, which are mediated by regulatory (or suppressor) cells (CD4+CD25+Foxp3+), or so called Treg cells, in the immune system. Treg cells secrete antiinflammatory interleukin-10 (IL-10) and IL-35. HT patients have reduced or inadequately functional regulatory cells [9]. Suppressor T lymphocyte dysfunction has been suggested to be important in the pathogenesis of this event and the pathology has been thought to be caused by an impairment in immune tolerance [10-12]. A reduced number of cells with suppressor properties leads to a reduction in the tolerance of the organism to self-tissue antigens. As a result of this defect, suppressor CD8+ T lymphocytes cannot suppress helper CD4+ T lymphocytes. Activated helper CD4+ T lymphocytes interact with B lymphocytes and these activated B lymphocytes then produce antibodies against several thyroid antigens [13,14].
IL-35 is a newly described cytokine with immunosuppressive and antiinflammatory effects [15]. In recent studies, the role of IL-35 in autoimmunity has been demonstrated and IL-35- expressing Treg cells and IL-35 were required for an optimal suppressive effect [16]. In the literature review conducted herein, a limited number of studies were encountered on the relationship between IL-35 and HT.
It was therefore aimed in this study to examine the IL-35 levels and their relationship with thyroid function tests.
MATERIAL AND METHODS
Study population
This study was planned at the Department of Internal Diseases of the Turgut Ozal University Faculty of Medicine. The study was begun following ethics committee approval dated 13/09/2012 and numbered B30 2 FTH 0200000/1099. Included in the study were 128 individuals, between 18 and 65 years of age, who had been newly diagnosed with HT (euthyroid, subclinical hypothyroidism, and overt hypothyroidism) and 38 health controls who had no known diseases or drug use.
Those who were 65 years of age, pregnant, had congenital hypothyroidism, hypothyroidism secondary to surgery, hypothyroidism secondary to drug use, evidence of a known acute or chronic infection, malignancy, acute-chronic kidney failure, chronic liver failure or did not sign the informed consent were excluded from the study.
While establishing the diagnosis of HT, clinical findings, physical examination, positive autoantibodies [elevated antithyroglobulin (anti-TG) (>34 IU/mL) and/or antithyroid peroxidase (anti-TPO) (>115 IU/mL)], and ultrasonographic findings (heterogenous appearance of thyroid parenchyma and reduced echogenicity) were taken into consideration, and a biopsy was not considered necessary. The period that was compensated by a mildly elevated thyroid stimulating hormone (TSH) (4.5 mU/L-10 mU/L) and when minor symptoms were observed only in some patients was defined as subclinical hypothyroidism, whereas the period when the TSH were elevated above 10 mU/L and the symptoms became more prominent was defined as overt hypothyroidism.
Sociodemographic characteristics, personal histories, family histories, and smoking-drinking and other habits of the patients and healthy volunteers included in the study, as well as the presence of a systemic disease or history of drug use, were investigated.
Biochemical analysis
For analysis of the biochemical parameters and studying the IL-35 levels of all of the participants, following a 12-h fasting, one tube of blood sample was collected from the antecubital vein before 10:00 A.M. After the collected blood samples were centrifuged at 5000 rpm, the serum and plasma samples were separated. After that, following the collection of blood samples of all of the participants, the IL-35 levels were studied at the same laboratory. Fasting blood glucose, routine biochemical work-ups, and lipid profile were measured spectrophotometrically using a Roche Cobas C501 device (Roche Group, Basel, Switzerland). Fasting insulin, free triiodothyronine (T3) (sT3 3), free thyroxine (T4) (sT4) and TSH levels were measured via the Enzyme Chemiluminescence Immunoassay (ECLIA) method in a Roche Cobas 6000 module device. Anti-TG and anti-TPO were studied via the ECLIA method in a Roche Cobas e 601 device.
IL-35 measurement
Serum IL-35 levels were measured via the sandwich ELISA method using the Cusab?o Biotech commercial kit (Cusabio Technology LLC, Houston, TX, USA; Catalogue no: CSB-E13126h). The sensitivity of this method was 15.6 pg/mL, intra-assay precision was <8%, and inter-assay precision was <10%, respectively.
Ultrasonographic examination
The HD15 PureWave Ultrasound System (Philips Medical Systems, Bothell, WA, USA) was used for ultrasonographic evaluation of the thyroid gland.
Statistical analysis
For the statistical analyses, IBM SPSS Statistics for Windows 20.0 (IBM Corp., Armonk, NY, USA) was used. Conformity of the variables to normal distribution was examined using the ShapiroWilk test. Descriptive analyses were given as the mean ± standard deviation for normally distributed variables and as the median and minimum-maximum values for non-normally distributed variables. Homogeneity of the groups and inter-group difference analyses were performed using one-way ANOVA and the least significant difference post-hoc test. For the inter-variable correlation analysis, the Pearson correlation tests were used. P < 0.05 was considered as statistically significant.
RESULT
Sociodemographic and laboratory characteristics of the groups are represented in Table 1;
Table 1: Clinical demographic and laboratory findings of the study population.
| Variables | Control n: 38 | Euthyroid n: 59 | Subclinical n: 39 | Overt hypothyroidism n: 30 | P-value | ||||
| F/M (n) | 28/10 (38) | 47/12 (59) | 30/9 (39) | 23/7 (30) | 0.927 | ||||
| mean | ± SD | mean | ± SD | mean | ± SD | mean | ± SD | ||
| Age | 39.29 | 12.99 | 39.83 | 11.47 | 40.05 | 11.58 | 36.30 | 11.63 | 0.544 |
| BMI | 27.76 | 3.94 | 25.45 | 3.31 | 27.52 | 4.92 | 25.27 | 4.01 | 0.006 |
| Smoking (n) | 2 | 2 | 2 | 1 | 0.952 | ||||
| HT (n) | 2 | 9 | 7 | 5 | 0.363 | ||||
| DM (n) | 3 | 7 | 6 | 6 | 0.161 | ||||
| mean | ± SD | mean | ± SD | mean | ± SD | mean | ± SD | ||
| TSH (uIU/mL) | 1.70 | 0.67 | 1.71 | 0.78 | 7.59 | 1.53 | 27.17 | 27.13 | 0.000 |
| ST4 (ng/dL) | 1.18 | 0.16 | 1.23 | 0.28 | 1.00 | 0.15 | 0.92 | 0.17 | 0.000 |
| ST3 (pg/mL) | 2.91 | 0.45 | 2.98 | 0.38 | 2.84 | 0.33 | 2.73 | 0.32 | 0.035 |
| TPOAb (IU/mL) | 10.8 | 12.8 | 479.2 | 495.1 | 402.2 | 379.5 | 457.6 | 371.1 | 0.000 |
| TG Ab (IU/mL) | 7.04 | 4.95 | 146.7 | 265.9 | 101.9 | 119.0 | 214.4 | 246.1 | 0.002 |
| TC (mg/dL) | 204.33 | 44.3 | 201.48 | 45.83 | 207.76 | 36.82 | 195.02 | 37.48 | 0.732 |
| TG (mg/dL) | 139.67 | 73.10 | 106.35 | 51.90 | 132.59 | 76.70 | 108.36 | 48.66 | 0.058 |
| HDL (mg/dL) | 50.55 | 12.97 | 56.63 | 15.46 | 51.13 | 12.83 | 53.62 | 9.14 | 0.152 |
| LDL (mg/dL) | 125.89 | 36.89 | 123.51 | 44.49 | 130.24 | 35.14 | 118.15 | 34.79 | 0.701 |
the groups were similar with regards to gender, age distribution, smoking status, number of individuals with hypertension, and diabetes mellitus (DM). The body mass index (BMI) was similar between the healthy control group and the subclinical hypothyroidism group; however, the levels in these groups significantly differed from the euthyroid and overt hypothyroidism groups (P = 0.006). When the patient groups and the control group were compared, the groups were similar in terms of total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride levels.
Although there was no significant difference between the groups in terms of IL-35 levels (P = 0.285), it was observed in the in-group comparisons that the IL-35 levels decreased progressively towards overt hypothyroidism. In the in-group comparisons, however, a statistically significant difference was determined between the control group and the overt hypothyroidism group (550.05 ± 411.50 vs. 369.80 ± 253.33; P = 0.046) (Table 2).
Table 2: IL-35 levels of the study groups.
| Control n: 38 |
Euthyroid n: 59 |
Subclinical n: 39 |
Overt hypothyroidism n: 30 |
|||||
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | |
| IL-35 level | 550.05 | 411.50 | 486.84 | 360.83 | 475.17 | 400.53 | 369.80 | 253.33 |
| Full Group | CG EG | CG SCHG | KG OHG | EG SCHG | EG OHG | SCHG OHG | ||
| P-value | 0.285 | 0.408 | 0.372 | 0.046 | 0.878 | 0.156 | 0.238 | |
When the patient groups were grouped according to their TSH values, a significant difference in the IL-35 levels was determined between the groups, with a threshold value of ≥6 uIU/mL and that below it (P = 0.043). When two groups were created, comprising the group with a threshold value of ≥10 uIU/mL and that below it, it was determined that the IL-35 levels more significantly differed between the groups (P = 0.024) (Table 3) (Figure 1).
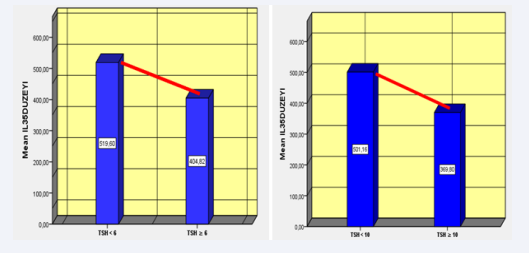
Figure 1 Relationship with IL-35 according to the TSH ≥6 and TSH ≥10 levels.
Table 3: Relationship with IL-35 according to the TSH ≥6 and TSH ≥10 levels.
| TSH | N | Mean | ± SD | P-value | |
| IL-35 level (ng/dL) | ≥6 | 61 | 404.81 | 327.54 | 0.043 |
| <6 | 105 | 519.60 | 384.58 | ||
| ≥10 | 30 | 369.80 | 253.33 | 0.024 | |
When the correlation analyses of IL-35 levels with the thyroid function tests were performed, a negative correlation of the IL-35 levels with the TSH, sT4 , and anti-TG levels was determined; however, no statistically significant difference was obtained. When the correlation analysis was performed by taking into account the controllable factors (smoking status, DM, hypertension, and BMI), a low-significant correlation was determined between the IL-35 and anti-TPO (P = 0.029) (Table 4).
Table 4: Correlation relationship between the IL-35 levels and thyroid function tests.
| TSH | sT4 | sT3 | Anti-TPO | Anti-TG | ||
| IL-35 level | r P-value |
–0.050 0.524 |
–0.072 0.355 |
0.025 0.748 |
0.152 0.051 |
–0.099 0.202 |
| Controllable variables (smoking status, DM, hypertension, BMI) | r P-value |
–0.063 0.430 |
–0.067 0.404 |
0.052 0.518 |
0.175 0.029 |
–0.096 0.232 |
DISCUSSION
While among the HT groups the IL-35 levels decreased progressively from the euthyroid group towards the overt hypothyroidism group in this study, a statistically significant difference could not be reached between the groups. The IL-35 levels were determined to be higher in the control group when compared to the overt hypothyroidism group. No significant difference was determined between the IL-35 levels and the thyroid function tests and autoantibodies.
Considering that IL-35 is an antiinflammatory cytokine that is secreted by Treg cells, it was hypothesized that it may be involved in development of HT. Because HT is an autoimmune disease and lymphocytic infiltration of the thyroid gland is crucial in its histopathogenesis, a potential reduction in the secretion of IL35, due to a reduced number and the function of Treg cells in HT and consequently, reduced antiinflammatory effectiveness, may be associated with lymphocytic infiltration of the thyroid gland. Thus, the role of IL-35 in autoimmunity has been demonstrated in recent studies [16], and it was shown in different experimental model systems that adaptively transferred iTR35 cells have effectively suppressed autoimmune diseases. In addition, in in vivo mice models, it has been demonstrated that, when IL35 derivatives were obtained from natural Treg cells, these cells induced iTR35-secreting cells while suppressing T cells. It has been proven in different studies that IL-35 regulates T cell activity and the suppression of T cell proliferation following the administration of recombinant IL-35 [18].
In this study, the IL-35 levels in HT we examined. No significant difference was determined between the groups in HT, although a reduction in the IL-35 levels with a progression towards the overt hypothyroidism group was observed in the in-group comparisons. In the in-group comparisons, however, a statistically significant difference was determined between the control group and the overt hypothyroidism group.
Hypothetically, a reduction in the IL-35 levels was expected with progression towards the overt hypothyroidism in HT. Hence, in this study, the reduction of the IL-35 levels with progression towards the overt hypothyroidism group, although not statistically significant, supported this hypothesis. When the patient groups were re-created using a threshold value for TSH, the resulting outcome became another point that supported this hypothesis. Nevertheless, when two groups were created by taking the threshold value for TSH as 6, <6 uIU/mL and ≥ 6 uIU/mL, it was observed that the IL-35 levels were statistically significantly reduced in the group with TSH ≥6 uIU/mL. When the groups were re-created using the TSH value as 10; however, it was found that the statistically significant reduction became more prominent. Based on this result, it can clearly be stated that the IL-35 levels reduced with progression towards manifestations of overt hypothyroidism in HT and that the development of an autoimmune process and the progression of the autoimmune process in HT may be closely associated with the reduction of the IL-35 levels.
In the literature, although there are studies regarding the IL35 levels in experimental models and animal studies, the number of studies regarding human serum IL-35 levels in autoimmune diseases is insufficient.
The small number of patients and cross-sectional design of this study were the major limitations. Moreover, the failure to study additional inflammatory parameters to determine the relationship of the IL-35 levels with antibodies in the HT group was another limitation.
In conclusion, in this study, it was determined that the IL-35 levels, an antiinflammatory cytokine involved in HT, decreased progressively from the euthyroid patient group towards the overt hypothyroidism group. For a better understanding of whether the IL-35 levels are involved in etiopathogenesis of HT and whether they have a relationship with autoantibodies, studies with a higher number of participants are required.









































































































































