Rapid Levothyroxine (Lt4) Absorption Test for Diagnosis of Lt4 Pseudomalabsorption: Case Report and Proposal of a Cutoff Point
- 1. Department of Prosthodontics, Hospital Universitário Onofre Lopes, Brazil
- 2. Department of Clinical Medicine, Universidade Federal do Rio Grande do Norte, Brazil
Abstract
Introduction: Levothyroxine pseudomalabsorption is a term used to describe a factitious disorder in patients with uncontrolled hypothyroidism despite the administration of high doses of levothyroxine (LT4), due to non-compliance.
Case: We present a 32-year-old female patient with severe hypothyroidism despite the daily use of 250 µg of LT4. She was submitted to the rapid absorption test of thyroid hormones, with supervised intake of 1000µg of LT4 and serial measurement of serum TSH and FT4, confirming the status of pseudomalabsorption. The patient underwent a psychiatric evaluation and was diagnosed as having a bipolar affective disorder with a current episode of severe depression, and a personality disorder with emotional instability. The patient was treated with mood stabilizers and supervised daily doses of LT4, and a significant improvement in clinical and laboratory was achieved. We analyzed 19 patients with LT4 malabsorption published in the literature and, compared to baseline, the minimum increase in FT4 was 2.5 times.
Discussion: Patients presenting refractory hypothyroidism despite the use of adequate doses of oral LT4 represent a challenge in clinical practice. In this setting, it is essential to establish the differential diagnosis of malabsorption syndromes, the presence of anti-T4 antibodies, causes of altered LT4 pharmacokinetics, and pseudomalabsorption. The LT4 absorption rapid test may be useful and an increase of at least of 2.5 times the baseline FT4 is suggestive of pseudomalabsorption.
Keywords
• Hypothyroidism
• Thyroid hormones
• Oral test
• Pseudomalabsorption of levothyroxine
Citation
Soares RMV, de Figueiredo RM, Melo Dantas MN, Solano Brito MV, Pires Sousa AG, et al. (2016) Rapid Levothyroxine (Lt4) Absorption Test for Diagnosis of Lt4 Pseudomalabsorption: Case Report and Proposal of a Cutoff Point. J Endocrinol Diabetes Obes 4(1): 1083.
ABBREVIATIONS
LT4: Levothyroxine; FT4: FreeT4; SLE: Systemic Lupus Erythematosus; VR: Values of Reference
INTRODUCTION
Hypothyroidism is a common endocrine disorder, affecting up to 8% around the world [1]. In most patients, hypothyroidism is readily treated with oral levothyroxine (LT4) replacement, with doses ranging from approximately 0.8-2.1 µg/kg [2]. In some cases, however, hypothyroidism is refractory to replacement, generally due to poor therapeutic compliance or reduced absorption of the drug [3]. Pseudomalabsorption of LT4 is a factitious disorder in which patients have hypothyroidism despite high doses of levothyroxine, due to non adherence. These patients differ from those with simple therapy abandonment because they usually have an underlying psychiatric disorder [4]. The aim of this article is to describe the LT4 absorption rapid test in a patient with hypothyroidism, highlighting its importance in the diagnosis of LT4 pseudomalabsorption.
CASE PRESENTATION
A 32-year-old woman was referred to the endocrinology clinic because hypothyroidism of difficult control. She was submitted to near-total thyroidectomy to treat papillary thyroid carcinoma in 2013, and histopathology revealed a 0.5cm papillary micro carcinoma without extra thyroid extension. The thyroidectomy was uneventful, and the patient did not need any additional treatment with ablation with I¹³¹.
Ever since, she has needed increasing doses of oral LT4, without reaching the euthyroidism. At the time of the first consultation, she reported asthenia, excessive sleepiness, myalgia, loss of recent memory, weight gain, constipation, nausea, vomiting, slow speech and reasoning, hair loss and amenorrhea. On physical examination, myxedema facies, periorbital edema, rarefied hair, and muffled heart sounds were presented.
The patient was also being treated for fibromyalgia, depression and arthralgia. She was on LT4 200µg/day, lorazepam 2.5 mg/day and omeprazole 20 mg/day. Arthralgia was being treated as systemic lupus erythematosus (SLE), and she also used prednisone 5mg/day, and hydroxychloroquine 400 mg/day.
Blood tests had shown a microcytic and hypochromic anemia (hemoglobin 9.4 g/dL), and thrombocytopenia (148.000/mm3 ), mild increase in aspartate aminotransferase (62mg/dL; VR <35mg/dL). The serum TSH was 286 mIU/mL, free T4 (FT4) <0.2 ng/dL and the anti-thyroglobulin antibody were negative. The serum thyroglobulin was undetectable, despite the TSH stimulation. Magnetic resonance imaging (MRI) of pituitary showed a diffuse enlargement of the pituitary gland, without mass, and with the stalk centered and of normal caliber.
Patient and her husband denied failure in compliance to take the LT4. She was advised to withhold omeprazole and increase LT4 dose to 250 µg/day. Antibodies against endomysium, transglutaminase, and parietal cells were negative.
After three months, there was no improvement in thyroid function tests. She presented episodes of syncope and was admitted for investigation of refractory hypothyroidism. During hospitalization, drugs that could interact with LT4 were excluded. Levothyroxine was administered at a supervised dosing by nursing. After rheumatology evaluation, the diagnosis of SLE was excluded, and prednisone and hydroxychloroquine were discontinued. A colonoscopy and upper endoscopy were performed, without abnormalities.
To evaluate the hypothesis of pseudomalabsorption, the patient was submitted to LT4 absorption test. After an overnight fasting, it was administered 1000 µg of LT4 (ten tablets of 100 µg each), with inspection of the oral cavity and observing behavior for 60 minutes. We measured TSH at 0, 1 and 2 hours and FT4 at 0, 30’, 45’, 60’, 120’ and 360’ after administration of LT4. The patient remained under medical observation for 12 hours and did not report any complaints. The results of TSH and FT4 are shown in (Table 1) and were compatible with normal absorption.
It was requested assessment of psychiatry, who diagnosed bipolar disorder with a current episode of severe depression, as well as emotionally unstable personality disorder. The psychiatrist started treatment with lithium carbonate and quetiapine.
During hospitalization, she remained with assisted administration of LT4, and the daily doses of LT4 were reduced until the patient be discharged on 125 µg. The patient referred reversal of clinical symptoms of hypothyroidism, and the last laboratory tests were TSH 11.5 mIU/mL and FT4 0.71 ng/dL.
DISCUSSION
Thyroid hormone replacement is one of the most effective hormone replacements of endocrinology since most patients achieve clinically and laboratory compensation with daily use of oral LT4, with good safety and tolerability. In most cases, the euthyroid state is obtained with a daily dosage ranging from 1.6 to 1.8 µg/kg [2]. The tablets are available in multiple dosages and should be taken orally in fasting. In cases of thyroid cancer, the therapeutic goal is the suppression of TSH, and patients use a higher dose (2.2 µg/kg/day) [2-3,5].
Approximately 62% to 81% of the ingested LT4 absorption occurs primarily in the duodenum and jejunum, within three hours of oral ingestion. The low gastric pH is critical for optimal dissolution of the drug particles and proper ionization of thyroxine [6-7]. Then the drug undergoes hepatic metabolism and goes to the biliary excretion of conjugated T4 and T3, which are partially deconjugated and resorbed in the intestinal tract [8].
Patients with clinical and laboratory uncontrolled thyroid function, even in the use of adequate doses of oral LT4, represent a challenge in clinical practice. In these cases, it is essential to establish the differential diagnosis of the presence of anti-T4 antibodies, malabsorption syndromes, causes of altered LT4 pharmacokinetics, and poor adherence (LT4 pseudomalabsorption).
The anti-T4 antibody may explain the therapeutic failure by interfering with the absorption of thyroid hormone. Its presence is related to treatment with thyroid dissected, making this a rare condition with only a few cases described in the literature [9].
The differential diagnosis of malabsorptive syndrome and pseudomalabsorption is complex. Exclusion of the causes that influence the pharmacokinetics of LT4 and perform the thyroid hormones absorption test can be quite useful.
Due to the importance of gastric pH for absorption of the drug, the proton pump inhibitors (PPI), as well as gastrectomy and gastric bypass can cause malabsorption [10]. In addition to the PPI, other drugs can also impair absorption of the LT4, especially aluminum hydroxide, ferrous sulfate, cholestyramine, and calcium [7]. Rifampicin, phenytoin, phenobarbital, and carbamazepine can accelerate the metabolism of thyroxine [11, 6,12,3,13,7,5]. Dietary factors (fiber, grape, soy and coffee) may also impair the LT4 absorption [7]. Gastrointestinal diseases such as celiac disease, atrophic gastritis, and lactose intolerance have interference at the hormone absorption [14].
We ruled out all of these causes in our patient and, after this, confirmation of pseudomalabsorption was done with LT4 absorption test. It can follow a six-hour protocol (rapid test) or up to six weeks (longer-term test) [10]. There are some reports in the literature with similar protocols, but usually, they are not standardized, and there are differences regarding the time of blood collection and measurement of TSH, free T4 or total T4 [11-13, 3]. We made some changes to the protocol proposed by Koulouri et al., [10] trying to combine both, fast and longer term tests. First of all in the pre-test phase, confounding factors (diet, medication and/or diseases) should be excluded. Then, in the rapid test phase, the cumulative weekly dose of LT4 is administered to the patient after an overnight fasting, and blood samples are collected at 0, 30’, 45’, 60 ‘, 90’, 120’ 240’ and 360’. Finally, the post-test phase consists of a supervised oral administration of the weekly dose of LT4 for five weeks with measurement of thyroid function tests in weeks 2, 3, 4, 5 and 6. Normalization of TSH confirm poor compliance, and a small increase of FT4 indicate a real malabsorptive syndrome [10].
In most cases reports of pseudomalabsorption published in the literature (Table 1),
Table 1: Age, gender and results of rapid absorption test (FT4 and TSH) after 1000µg of oral LT4.
| Author | Number of patients | Age (yrs) | Gender | Free T4 (ng/dL)a | TSH (mIU/mL) | ||||||||||||
| 0 | 30 | 60 | 120 | 240 | 360 | RV | FT4 peakb | 0 | 30 | 60 | 120 | 240 | 360 | ||||
| Our patient | 1 | 32 | F | 0.20 | 0.20 | 0.27 | 1.60 | - | 1.49 | 0.89-1.76 | 8.0 | 340.0 | 330 | 149 | |||
| Livadariu [8] | 1 | 69 | F | 0.08 | - | - | 1.52 | 1.55 | 1.46 | 0.70-1.70 | 19.3 | 59.7 | - | - | 61.2 | 55.6 | 56.3 |
| Pedrosa[17] | 1 | 49 | F | 0.20 | - | - | 1.21 | 1.10 | - | 0.75-1.80 | 6.0 | 351 | - | - | 272 | 261 | - |
| Srinivas[5] | 1 | 55 | F | 0.56 | - | - | 2.66 | 2.23 | 2.19 | 0.93-1.71 | 4.75 | 65.7 | - | - | 58.4 | 53.2 | 51.2 |
| Lips[13] c | 1 | 33 | F | 0.68 | - | - | 2.10 | 1.86 | 1.83 | - | 3.0 | - | - | - | - | - | |
| Goichot[19] c | 1 | 55 | M | 0.66 | 1.52 | - | 1.94 | - | - | 0.71-1.85 | 2.9 | 108 | 87.5 | - | - | - | - |
| Abel [20]c | 1 | 33 | F | 0.49 | - | - | 2.50 | 2.10 | - | 0.85-1.69 | 5.1 | 57.2 | - | - | 27 | 23 | - |
| Payer [21] | 1 | 42 | F | 0.28 | - | - | - | 0.70 | - | - | 2.5 | 126 | - | - | - | 75 | - |
| Eledrisi [16] | 1 | 41 | F | 0.76 | - | - | 2.05 | 2.06 | 2.69 | - | - | - | - | - | - | - | |
| Sun [18]d | 10 | 40.5 | 7F 3M |
0.50 0.20 1.60e 0.50 0.10f 0.90 0.40 0.10 1.00 0.80 |
2.30 1.50 2.70 1.80 0.10 2.10 2.00 1.20 2.20 2.10 |
0.70-1.80 | 4.6 7.5 1.68 3.6 0 2.3 5.0 12 2.2 2.62 |
17 295.3 6.8 94.1 122.8 73.7 54.1 180.8 23.2 38.7 |
|||||||||
| All patients receive 1000 µg orally just before test. a The values previously described as pmol/L were converted to ng/dL using the conversion factor 0.07769. b Relative increase in the peak FT4 compared to baseline values. c Values based on the graphical analysis. d Report of the values found in the retrospective analysis of ten oral LT4 absorption tests. e Patient with biopsy-proven celiac disease. f Patient who had an abnormal absorption result. | |||||||||||||||||
the rapid absorption test was conducted by measuring serum levels of TSH, free T4, and TT4 (in some cases) before and after 2, 4 and 6 hours of supervised LT4 ingestion. The most commonly used dose is 1000 µg, in liquid or as tablets [15-16, 13,8,3,17,5,18]. The observation of the patient for at least 60 minutes after ingestion is important to evaluate if the patient develops any symptoms and to ensure the compliance [10]. There is no data in normal people, but in patients with hypothyroidism, the FT4 usually increases 1 hour after LT4 intake and achieves a peak at 2 hours [11]. Despite the elevation in FT4 makes LT4 malabsorption unlikely, the FT4 cutoff reference values accepted by all are not known, making difficult the test interpretation.
Some authors suggest the calculation of the volume of distribution (Vd) to estimate the amount of drug absorbed. The Vd is measured in liters and obtained by calculating: Vd = 0,442xBMI. Based on this, the percentage of drug absorption can be estimated by the formula: LT4 absorbed (%) = peak TT4 (µg/ dL) x Vd (dL)/ administered dose of LT4 x 100. Besides not being easy to use, this formula uses total T4. The dosage of the FT4 in association with TSH is widely recommended in the evaluation and management of hypothyroidism because it suffers less influence of intrinsic and extrinsic factors when compared to the total T4 [18].
With the purpose of evaluating the use of the FT4 in the oral LT4 absorption test, a recent study compared utilization of total or free T4 after 1000µg of LT4 [18]. The authors found a strong correlation between measures of total T4 and FT4 (r = 0.88, p<0.001), suggesting that FT4 may be used instead of total T4 [18].
There was a significant increase in the levels of FT4 throughout the test of 19 LT4 pseudomalabsorption patients (42.8±8.8 years; 15 female) published in the literature (Table 1). The mean baseline FT4 was 0.52±0.3 ng/dL and the median of TSH was 73.7 (6.8-351.0) mIU/mL. Some patients had already used the weekly supervised dose of LT4 and had TSH only slightly elevated and normal FT4. The FT4 peak commonly occurs after two hours and, considering all patients, it was 4.7 (1.7-19) times the baseline value. If we consider only patients with baseline FT4 below the lower limit of reference values, the median FT4 increase was 5.0 (2.5-19) times, and all patients increased at least 2.5 times the baseline value (Figure 1),
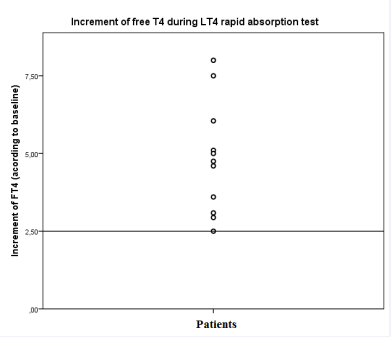
Figure 1 Increment of Free T4 (FT4) after 1000µg of oral levothyroxine (LT4) in the rapid absorption test. Only patients with baseline FT4 bellow the lower limit of reference were included. All patients had an increment of 2.5 or higher.
suggesting a normal absorption of the LT4. TSH was not available for all cases and did not decrease substantially. Therefore, we suggest the test could last no more than two hours and, in the absence of optimal cutoffs previously established, an FT4 increase of at least 2.5 times the baseline is simple criteria to confirm the proper absorption of LT4. The adoption of this simplification may facilitate and amplify the use of the rapid LT4 absorption test, and reduce costs by measuring only FT4.
Our results are limited by the lack of a control group with malabsorption syndrome, to better determine sensitivity and specificity of the cutoff FT4 value using ROC curve analysis. Reports of patients with malabsorption syndrome who underwent oral test LT4 absorption are not available.
The treatment of pseudomalabsorption becomes more difficult if the patients psychiatric disorders are not diagnosed and treated. The most common psychiatric disorders include depression, Munchausen syndrome, and factitious disorder [17]. The supervised administration of LT4 is considered an effective and less invasive therapeutic measure. Since the psychiatric disorder is confirmed, appropriate psychiatric supervision is essential to reduce the risk of relapse. Informing the patient and relatives about the effects of poor compliance to treatment can often improve adherence [5]. However, this therapeutic approach can be difficult and requires the interaction of clinical and psychiatric teams, in addition to the patient and his/her family [13,17].
In conclusion, we report a case of pseudomalabsorption of LT4 with its diagnostic challenges, highlighting the importance of the LT4 absorption test to the diagnostic investigation in addition to the need to exclude the factors that can interfere with the replacement therapy. We emphasize that the benefit of using LT4 absorption test is clear in these cases and, although clinical studies are needed to determine a standard protocol with appropriate cutoffs, we suggest that an increase of at least of 2.5 times the baseline FT4 is suggestive of pseudomalabsorption.









































































































































