The Cardiovascular Effects of Metformin: Conventional and New Insights
- 1. Department of Internal Medicine and Bioregulatory Science, Kyushu University, Japan
- 2. Innovation Center for Medical Redox Navigation, Kyushu University, Japan
Citation
Batchuluun B, Sonoda N, Takayanagi R, Inoguchi T (2014) The Cardiovascular Effects of Metformin: Conventional and New Insights. J Endocrinol Diabetes Obes 2(2): 1035.
INTRODUCTION
Metformin, a first-line agent for type 2 diabetes pharmacotherapy, is one of the most prescribed drugs worldwide. Its glucose lowering effect is mediated through suppressing the hepatic production of glucose, decreasing intestinal glucose absorption and improving glucose uptake and utilization [1]. Despite the emergence of new glucose lowering medications, metformin is still reported to be the agent most widely used to treat diabetes. This is because of its safety record over past decades and its various beneficial outcomes, as well as its glucose lowering action. One of the most favorable effects of metformin is cardiovascular protection in diabetes. This protective action is vital in diabetic patients because microvascular and macrovascular diseases are responsible for most diabetes-associated morbidities and mortalities [2]. Identifying the molecular targets of metformin is likely to enable the development of second-generation drugs with similar properties, including the prevention and management of diabetic vascular complications. In this review, we summarize conventional and new understandings of the effects of metformin on cardiovascular outcomes and its mechanisms of action involving cardiovascular protection.
Metformin and cardiovascular outcomes
The history of metformin is linked to the use of Galega officinalis as an herbal medicine in medieval Europe. Metformin was first synthesized in 1929 and the first clinical trial of metformin as a treatment for type 2 diabetes was published in 1957 [3,4]. Since then, metformin has been thoroughly researched in diabetes, with large clinical trials investigating the outcomes of treatment with this agent. The UK Prospective Diabetes Study (UKPDS) showed that early intervention with metformin in patients with type 2 diabetes decreased the incidence of diabetes-related vascular endpoints by 32%, myocardial infarction by 39%, diabetes-related deaths by 42% and all-cause mortality by 36% [5]. In addition, the rates of diabetes-related endpoints and all-cause mortality were significantly lower in patients receiving metformin than in patients treated with sulfonylureas or insulin. Subsequent trials yielded similar findings, confirming that metformin has a protective effect against cardiovascular out comes in type 2 diabetes [6-10]. A trial in diabetic patients with a previous myocardial infarction showed that metformin treatment resulted in a lower mortality rate than sulfonylurea treatment [11,12]. In addition, metformin treatment of type 2 diabetic patients for 2–3 years reduced carotid intima-media thickness (CIMT) [13,14], and an observational study showed that the metformin-associated reduction in cardiovascular morbidity and mortality was independent from its glucose lowering properties [15]. The ability of metformin to provide similar cardiovascular benefits to non-diabetic individuals was also tested [16]. In that study, metformin did not significantly affect the progression of CIMT, carotid plaque score, and several other markers of cardiovascular disease, indicating that metformin has no effect on CIMT in non-diabetic patients with high cardiovascular risk. However, metformin was shown to reduce CIMT in non-diabetic women with polycystic ovary syndrome [17] and to increase lower arterial flow in non-diabetic patients with peripheral vascular disease [18]. Thus, although metformin treatment clearly benefits diabetic patients, its benefits in non-diabetic patients are unclear.
Mechanism of the cardiovascular effects of metformin
Clinical evidence of the protective effects of metformin prompted evaluations of its mechanism of action and molecular-tar gets. Metformin was reported to improve lipoprotein profiles in diabetic patients by decreasing plasma concentrations of free fatty acid, triglycerides, total cholesterol and LDL cholesterol and by increasing HDL cholesterol [1,19]. Moreover, metformin was shown to decrease hypercoagulation and to increase fibrinolysis in insulin resistant states, apparently by reducing plasminogen activator inhibitor-1(PAI-1) [20]. In addition, metformin reduced platelet sensitivity to aggregation [21] and chronic low-grade inflammation in the atherogenic process [22]. In addition to its anti-atherogenic effects, metformin was reported to ameliorate cardiac functional abnormalities in rats with diabetic cardio- myopathy [23]. Because oxidative stress plays a pivotal role in vascular tissue damage, the cardiovascular protective effects of metformin may be because of its ability to prevent diabetes-induced oxidative stress, [24-27]. Hyperglycemia-induced protein kinase C (PKC) activation and subsequent activation of NAD (P) H oxidase is a pathway causing the overproduction of Reactive Oxygen Species (ROS) in diabetes [28-31]. PKC is associated with vascular alterations, such as increases in permeability, contractility, extracellular matrix synthesis, cell growth and apoptosis, angiogenesis, leukocyte adhesion, and cytokine activation and inhibition [30]. The PKCβ2 isoform is preferentially elevated in diabetic heart and aorta [28], whereas a specific inhibitor of PKCβ improved vascular dysfunction in diabetic rats [32] and clinical parameters of diabetic nephropathy in patients with type 2 diabetes [33], suggesting the significance of PKCβ activation in diabetic complications. A recent trial showed that metformin reduced oxidative stress parameters in patients newly diagnosed with type 2 diabetes [34], consistent with results showing that metformin inhibited diabetes-induced oxidative stress in endothelial cells [35,36]. Our recent study revealed that metformin suppressed high glucose-induced oxidative stress in human aortic endothelial cells by inhibiting the PKC-NAD (P)H oxidase pathway [37]. The ability of metformin to protect against cardiovascular outcomes may be because, at least in part, of these properties.
Molecular Targets of metformin
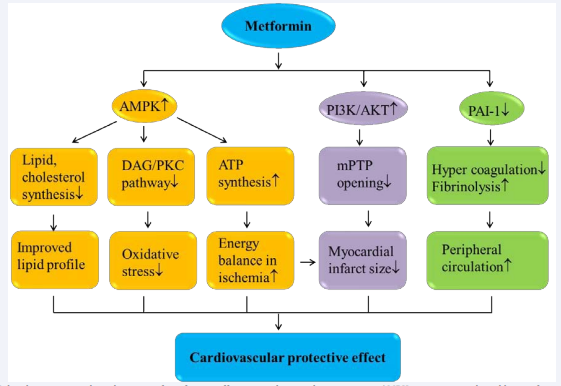
Identifying the molecular targets underlying the protective effects of metformin is important in the development of second generation drugs that may improve the management of diabetic cardiovascular complications. AMP-activated kinase (AMPK) is a target for the anti-hyperglycemic action of metformin, as well as playing a critical role in metformin-mediated cardiovascular protection (Figure 1).
Figure 1: Molecular targets and mechanisms of metformin effect on cardiovascular protection. AMPK activation mediated by metformin treatment improves lipid profile, energy balance and decreases oxidative stress. Metformin is also shown to decrease myocardial infarct size through PI3K/ AKT activation, and increase peripheral blood circulation by decreasing PAI-1. These properties of metformin may contribute?its positive impact on cardiovascular protection.
AMPK is a major regulator of cellular energy and is activated by an increase in the cellular AMP: ATP ratio during energy stress. AMPK was shown to maintain the energy balance in cells during ischemia by increasing ATP levels [38]. Metformin increased AMPK activation in both ischemic and non-ischemic hearts but failed to protect cardiac-specific AMPKα2 dominant-negative transgenic mice [39], suggesting that AMPK is essential for the protective effects of metformin. Similarly, rats treated with metformin showed a significant reduction in infarct size compared with untreated animals [40]. However, the metformin mediated reduction of infarct size also involves other potential target molecules, including the phosphatidylinositol-3-kinase (PI3K)-Akt pathway and adenosine concentration [41]. The mitochondrial permeability transition pore (mPTP) opens, leading to necrosis and apoptosis in reperfusion. It was reported that metformin reduces myocardial infarct size in both the non-diabetic and diabetic heart through preventing the opening of mPTP by activation of PI3K and Akt [42].
Metformin was also shown to increase fatty acid oxidation through AMPK activation, decreasing the amounts of intracellular free fatty acids available for the production of Diacylglycerol (DAG) and triacylglycerol. In diabetes, hyperglycemia increases the de novo synthesis of intracellular DAG, which consists of two fatty acid chains covalently bound to a glycerol molecule. De novo synthesized DAG acts upstream of the PKC pathway; therefore, increased DAG induces PKC activation, resulting in increased oxidative stress [28-31]. Our recent study showed that metformin treatment significantly increased AMPK phosphorylation and greatly reduced intracellular DAG subgroups, including 1-palmitoyl-2oleoyl-glycerol,1,2-dipalmitoyl-glycerol and 1,2-dioleoyl-glycerol. Moreover, metformin inhibited the PKC-NAD (P)H oxidase pathway, subsequently reducing high glucose-induced oxidative stress in aortic endothelial cells [37]. Thus, AMPK is likely to be crucial for the ability of metformin, not only to maintain energy homeostasis in ischemia but to prevent diabetes-induced oxidative stress.
Metformin and incretin combinations
In recent years, Glucagon-Like Peptide-1 (GLP-1) has emerged as a new potential anti-diabetic treatment option. GLP-1, an incretin hormone secreted by distal intestinal L cells, is important for the regulation of glucose homeostasis. In addition to its multi-organ targeted anti-diabetic effects, such as improved insulin secretion, reduced food intake, decreased glucagon secretion and increased mass of pancreatic β-cells [43-47], GLP-1 has been found to have protective effects on cardiovascular function [46- 48]. Metformin increases the endogenous secretion of GLP-1 and decreases the activity of DPP-4, an enzyme that degrades GLP-1, resulting in greater glucose lowering in type 2 diabetes [49- 52]. We recently found that, in addition to this glucose-lowering effect, metformin, at a therapeutic concentration, enhances the ability of a GLP-1 analog to prevent oxidative stress caused by high glucose concentrations [37]. Hyperglycemia-induced PKCβ2 activation was recently shown to reduce the expression of GLP-1 receptor protein in the glomerular endothelium, blunting the effect of GLP-1 [45]. Because metformin inhibits PKCβ2 signaling [37], the combination of metformin and GLP-1 analog may be preferable clinically because metformin prevents GLP-1 receptor degradation by inhibiting PKCβ2, improving the glucose lowering and cardiovascular protective effects of GLP-1 in patients with diabetes.
CONCLUSION
Metformin is a well-established component of diabetic management. In addition to its glucose-lowering effect, metformin has been found to provide various benefits, enhancing cardiovascular protection in diabetic patients as shown in large clinical trials, whereas the action of metformin in non-diabetic patients may be specific to certain conditions or individuals. For example, metformin has protective effects against cardiovascular risk in patients with polycysticovarian syndrome, with these effects being important in patients with advanced cardiovascular risk. The cardiovascular protective mechanisms of action of metformin may include improved lipid profiles, anti-atherogenic effects, decreased ischemic injury and amelioration of oxidative stress. Combining metformin and GLP-1 may be more effective in patients with type 2 diabetes than either alone.