The effect of Non-Caloric Restricted, Low-Carbohydrate Diet in Reversing Type 2 Diabetes Mellitus among Active Omani Diabetic Patients Attending North Mawaleh Health Center
- 1. North Mawaleh Health Center, Muscat, Oman
Abstract
Background: There is growing evidence that low-carbohydrate diet can positively improve glycemic index in patient with type 2 diabetes mellitus in compare with currently recommended method of low fat, high carbohydrate diet for the same group. However, more researches are needed to prove the effectiveness and safety of this type of diet before starting to implement it to diabetic patients.
Objective: This study focused on the effectiveness of non-caloric restricted, low-carbohydrate diet in improving glycemic control over 24-week period in active Omani diabetic patients attending primary care setting at North Mawaleh health center in Muscat Government.
Research design and method: This is prospective descriptive study with longitudinal follow up and pre-test, post- test comparison. Eighty-three patients were recruited. Blood collected at baseline, at 12-week and 24-week. Each patient was advised to follow non-caloric restricted low-carbohydrate regimen (< 80 gm of carbohydrate per day) and exercise recommendations. The patients were seen every alternate week in the first month then monthly to look for reflow measurements, counselling and medication adjustment. The primary outcome was glycated hemoglobin (HbA1c).
Result: Seventy-one patients were able to complete the study. Non-restrictive low-carbohydrate diet (< 80 gm of carbohydrate/day) showed marked reduction in glycated hemoglobin in 24-week period by 11.58%, from 7.12 (SD1.067) % at week 0 to 6.28(SD1.066) % at week 24, p value < 0.05. This reduction was noticed along with adjustment of diabetic medications. Additionally, the percentage of patients with optimal level of HbA1c (<7%) was improved from 58 % of total patients studied to 79 % by the end of the study. There was significant mean weight reduction by 7.33 % from 82.63(SD14.3) kg to 76.67(SD14.90) kg (p value <0.05). High density lipoprotein increased from 1.30(SD0.297) to 1.40(SD0.468) mmol/l, p value <0.05 and TAG was reduced from 1.47(0.934) to 1.22(0.564) mmol/l value <0.05. The mean eGFR was declined from 86.73 (SD6.21) to 84.92 (SD8.47) ml/minper1.73m2 at the end of the study (p value <0.05). Diabetic medications were stopped in 18 (25.4%) patients, reduced in 7(9.8%) patients, increased in patients 2(2.8%), and unchanged in 44(62.0%) patients. The linear regression failed to show any correlation between reduction in HbA1c and weight changes.
Conclusion: Non-caloric restricted LCD improved glycemic control in patient with type 2 DM. It also improved lipid profile, BMI and medication requirements. However, it demonstrated adverse effect on eGFR in patient with normal eGFR. Further controlled studies are warranted.
Keywords
• Low carbohydrate diet Gglycated Hemoglobin
Citation
Alkalbani S (2020) The effect of Non-Caloric Restricted, Low-Carbohydrate Diet in Reversing Type 2 Diabetes Mellitus among Active Omani Diabetic Patients Attending North Mawaleh Health Center. J Endocrinol Diabetes Obes 8(1): 1121.
INTRODUCTION
Low fat, high carbohydrate diet is the recommended dietary method in patient with type 2 diabetes mellitus. However, this type of diet has higher proportion of carbohydrate that can raise the postprandial serum glucose and subsequently increase insulin requirement. Furthermore, low-fat diet was linked to increase in the risk of developing cardiovascular diseases. In recent years, there was growing evidences that low-carbohydrate diet (LCD) can lead to improvement in glycemic index and reduced weight at short term. However, few well controlled studies have comprehensively examined its long-term effectiveness on glycemic control and cardiovascular disease (CVD).
Low carbohydrate diet
Low-carbohydrate diet is basically restricted caloric intake by reducing the consumption of carbohydrates to 20 to 60 gm per day (typically less than 20 percent of the daily caloric intake) as per American academy of Family Physician [1]. However, there is no widely accepted definition of what precisely constitutes a low-carbohydrate diet. It is important to note that the level of carbohydrate consumption defined as low carbohydrate by medical researchers may be different from the level of carbohydrate defined by dietitian. On the other hand, the consumption of protein and fat is increased to compensate for part of the calories that formerly came from carbohydrates. The Glycemic Index (GI) is a relative ranking of carbohydrates on a scale from 0 to 100 according to the extent to which they raise blood glucose levels after eating. Carbohydrates with a low GI value (55 or less) are more slowly digested, absorbed and metabolized and cause a lower and slower raise in blood glucose and, therefore insulin levels [1]. On the other hand, foods with a high GI are those which are rapidly digested, absorbed and metabolized and result in marked fluctuations in blood glucose levels. Thus, Low GI-carbohydrates are considered one of the secrets to long-term health, reducing risk of type 2 diabetes and heart disease. It is also one of the keys to maintaining weight loss. Thus, patients following low-carbohydrate diet have advantage of reducing glycemic index and subsequently improve blood glucose level. It is important to mention that ketosis readily occurs at carbohydrate intakes below 50 gm/ day which demonstrate that the body’s glycogen supplies have been consumed and that protein and fat are being used as fuel. In addition, very low carbohydrate, ketogenic diets (VLCKD) appear to have more pronounced effects than other, less restricted carbohydrate diets in controlling glycemic level. Furthermore, low-carbohydrate diets initially induce significant water diuresis due to glycogenolysis from increased protein consumption. Thus, a portion of the early weight loss in these diets is water weight.
In one study, where they compared different dietary approaches for the management of type 2 diabetes mellitus, they found that low carbohydrate diet (LCD) is effective in improving various markers of cardiovascular risk in people with type 2 DM [2,3]. Another study compared the effectiveness and safety of LCD (130 gm/day) with calorie restricted diet (CRD) in reducing HbA1c and BMI in Japanese patients with poorly controlled T2DM and it demonstrated significant outcome in LCD compared to the CRD [4]. Furthermore, A study showed low-carbohydrate ketogenic diet (<20 gm carbohydrate / day) decreased HbA1c from 7.5 (+/- 1.4) % to 6.1 (+/- 1), p=0.001, along with reduction and/or discontinuation of diabetic medication [5]. Another study compared the effect of low-carbohydrate, ketogenic diet versus low glycemic index diet on glycemic control in type 2 DM revealed that low carbohydrate diet had greater improvement in glycemic control, more frequent medication adjustment and elimination than low glycemic index diet [6]. However, the effect on renal function (creatinine clearance and eGFR were insignificant in several studies [5,7,8].
METHODOLOGY
Participants
Total of 83-participant were recruited which represents the active patients with type 2 diabetic mellitus who attended diabetic clinic at North Mawaleh health center (NMHC) from January 2017 to December 2018 and have met the inclusion and exclusion criteria. The inclusion criteria were: active diabetic patients aged 20-60 year who are registered in NMHC diabetic registry, diagnosis of type 2 DM made within last 8 years, diabetic patient on oral hypoglycemic agent, diabetic patient with no h/o diabetic ketoacidosis or end organ damage, diabetic patient with body mass index (BMI) more than or equal to 25 kg/m2 and no recent change in diabetic medication for the last three-month. The exclusion criteria were: diabetic patient on insulin therapy, previous history of diabetic ketoacidosis or end organ damage, chronic kidney disease stage 3 and less (eGFR < 60 ml/min per1 ·73m2 , active liver diseases or CVD, pregnant ladies with type 2 DM, or breastfeeding ladies with type 2 DM , and patient who fail to adhere to non-caloric restricted low-carbohydrate diet. All potential patients were called for office interview where they got a brief explanation and time frame of the study. Informed consent was taken for each participant before being recruited in the study. The patient had all the right to withdraw from study any time he/she wants.
Study design
This is prospective descriptive study with longitudinal follow up and pre-test post-test comparison undertaken in the outpatient clinic of North Mawaleh health center in Muscat Government over 24-week period from 01/01/2019 to 30/06/2019.Ethical approval was obtained from scientific research committee at ministry of health. All enrolled patients had signed consent form prior to entry to the study.
Sample size
Total of 83 participants were recruited. This number represents all active patients with type 2 diabetic mellitus who attend diabetic clinic since January 2017 till June 2018 and met the inclusion and exclusion criteria. The active patient with type 2 DM were defined as those who attend diabetic clinic at least 2 times per year and found to be 482 out of 1007 registered in the health center (taking in to consideration that this number represent total patient since the start of registration in 2010, but some of them were transferred, died or not regular follow up). Out of this number only 93 of those were fully met the inclusion and exclusion criteria and out of this number 83 signed the consent form to start on this study.
Intervention
After meeting the selecting criteria of the study, a baseline history, physical examinations and laboratory investigations (LFT, RFT, Lipid profile, Hb A1c, Albumin creatinine ratio, TSH, uric acid, ACR, FBS) were collected before the start of the study. Then, all participants were given detailed information about non-caloric restricted low-carbohydrate diet (< 80 gm of carbohydrate/day) by dietitian as individual or in small group sessions at first encounter. Along with proposed diet, patients were allowed unlimited amount of animal food and eggs, unlimited amount of unsaturated fatty acid, restricted amount of saturated fatty acid. Furthermore, they were provided with leaflet of proposed diet to follow-up through 24-week period. The patients were advised to keep diary of their diet and to submit it in each visit. In addition, everyone was instructed to followup exercise recommendation of at least 30 minutes of moderate intensity exercise for at least five times per week, but no formal exercise program was proposed.
The patients were followed every other week in the first month then monthly thereafter for anthropometric measurement (weight and BMI, waist circumference), vital signs measurement (BP, FBS), adherence to diet and exercise advice and medications adjustment. To maximize adherence to study visits, participants were provided with an appointment schedule and received appointment reminders (phone calls or text messages) before visits. Weight was measured by using electronic scale. Waist circumference was also measured by using measurement tap positioned 3 cm above iliac crest. Reflow measurement, using personal glucometer devices that were well calipered, was done every alternate day in order to plan for any adjustment in oral hypoglycemic medications (OHA). Medications (including the dosage and frequency) at baseline and change throughout the study period were documented. In each visit, participants were advised to present their diary of diet and exercise to check their adherence to proposed regimen. Additionally, direct questioning about hypoglycemic symptoms, craving for carbohydrate and other possible side effect were done at each visit. Those who fail to adhere to dietary regimen were excluded from the study. The laboratory investigations were done at 3 stages, baseline, 12- week and 24-week.
Outcome
Primary outcome: The primary outcome was HbA1c. It represents the control of blood glucose level over previous 3-month and thus it is an excellent predictor of primary outcome. It was measured at baseline, 12-week and 24-week by using immunoassay technique.
Secondary outcome: The secondary outcomes were lipid profile (TC, LDL, HDL, TAG), eGFR, weight changes and medication adjustment. The laboratory investigations were collected in the morning after fasting for at least 8 hours and processed at same laboratory center to ensure standardization of the results. Full laboratory investigations were done before the start of the study, at 12-week and 24-week. Moreover, weight was also checked at each encounter using calipered scale. Additionally, diabetic medications adjustment was also documented at each visit based on reflow measurement, adverse effect and diet and exercise compliance were also documented. Adherence to proposed dietary regimen was also assessed at each encounter and those who fail to adhere to it were excluded from the study. The full history, physical examination and laboratory investigations were taken at the end of 24-week period. Changes in all variables were analyzed by the paired t-test. Linear regression analysis was used to examine predictors of change in hemoglobin A1c. A p value of 0.05 or less was considered statistically significant.
Funding source
The researcher has conducted the study independent of any funding source. There was no rule for funding source in conducting the study.
RESULT
Study participant
Eighty-three participants had been recruited in this study, out of which 71 completed the full 24 weeks period of the study with 14 % discontinuation rate. The reasons for discontinuation were: 5 had difficulty to adhere to diet regimen and 7 were unable to attend the proposed meeting schedules due to other commitments. However, No one has reported that diet side effect as the main reason for discontinuation of diet regimen. The female participants accounted for 66.2 % (n=47) of total participants while the male participant accounted for 33.8% (n= 24). The baseline characteristic of the study is shown in the Table 1 below.
Table 1: Baseline characteristic (n=71).
| Characteristic | summary |
| Age, years, mean (SD) | 48.0(6.76) |
| Gender, female, n (%) | 47(66.2%) |
| Weight, kg, mean (SD) | 82.63(14.3) |
| Height, m, mean (SD) | 1.6(0.84) |
| BMI, kg/m2, mean (SD) | 31.28(5.49) |
Dietary adherence and physical activity compliance
All candidates were given written prescription of dietary regimen (low carbohydrate diet of < 80 gm/day) and physical activity advice to follow throughout the period of study. The patients were advised to keep diary of daily intakes and physical activities and present it in each visit to ensure their adherence to diet regimen. Patients who fail to adhere to dietary regimen were excluded from the study. Total of 12 (14.5%) patients dropped out of the study and of which 5 (41.6%) patients had difficulty to adhere to diet regimen.
Anthropometric measurement
The mean body weight had shown significant reduction by7.33 % from 82.63(SD14.3) kg at week 0 to 76, 67 (SD14.9) kg at the end of the study (p value <0.05) (Table 1-4).
Table 2: Anthropometric measurement.
| Measurement | Week 0 Mean (SD) | Week 12 Mean (SD) | Week 24 Mean (SD) | Mean changes (0-24 weeks) | p-value (0-24 weeks) |
| Weight | 82.63 (14.3) | 78.41 (14.5) | 76.67 (14.90) | -5.96(-7.33%) | <0.05 |
| Waist circumference | 105.5 (11.16) | 102.3 (11.16) | 101.01 (11.129) | -4.26% | <0.05 |
Table 3: The effect of non- caloric restricted low-carbohydrate diet on Glycated hemoglobin (HbA1c)..
| Hb1c % | HbA1c (0-week) | HbA1c (24-week) | p-value (0 to24 week) |
| <7% | 41(58%) | 56(79%) | <0.05 |
| 7 to 9% | 23(32%) | 14(20%) | |
| >9% | 7(10%) | 1(1%) |
Table 4: The effect of Non-caloric restricted low-carbohydrate diet on other metabolic measures.
| Measurement | Week 0 | Week 12 | Week 24 | Change (0to 6 Month) | p-value (0 to 24week) |
| Mean (SD) | Mean (SD) | Mean (SD) | % | ||
| Weight | 82.63 (14.3) | 78.41 (14.5) | 76.67 (14.90) | -7.33% | <0.05 |
| WC | 105.5 (11.16) | 0 | 101.01 (11.129) | -4.26% | < 0.05 |
| Hba1c | 7.12 (1.067) | 6.18 (1.17) | 6.28 (1.066) | -11.58% | <0.05 |
| TC | 4.26 (0.989) | 4.13 (0.745) | 4.22 (0.770) | 0.88% | .740 |
| LDL | 2.30 (0.943) | 2.26 (0.96) | 2.35 (0.709) | 2.24% | .577 |
| HDL | 1.30 (0.297) | 1.34 (0.300) | 1.40 (0.468) | 7.55% | <0.05 |
| TAG | 1.47 (0.934) | 1.15 (0.543) | 1.22 (0.564) | 15.71% | <0.05 |
| EGFR | 86.73 (6.21) | 85.56 (7.74) | 84.92 (8.47) | -2.1 % | <0.05 |
| ACR | 5.87 (13.89) | 6.0 (17.07) | 4.85 (14.61) | -16.96% | .428 |
| UA | 299.14 (72.34) | 316.67 (69.52) | 307.98 (72.51) | 3.12% | .182 |
| TSH | 2.28 (1.44) | 2.16 (1.51) | 2.31 (1.73) | 1.64% | .839 |
Thus, the net reduction of body weight at 24-week was – 5.96 kg. The waist circumference had also demonstrated significant reduction from 105.5 (SD11.16) cm at 0-week to 101.01(SD11.129) cm at 24- week (p value < 0.05).
The change in BMI at baseline and at 24 weeks was also noticed. At baseline, the distribution of patients as per their BMI were as following: 0% with normal weight( BMI 18-24.9 kg/ m2 ), 34 % with overweight( BMI 25-29.9 % kg/m2 ), 32 % with obesity class I (BMI 30-34.9 kg/m2 ), 25 % with obesity class II (BMI 35-39.9 kg/m2 ) and 8 % with obesity class III ( BMI 40 kg/ m2 and above). There were more pronounced changes noticed in patients with class I ( from 32% at week 0 to 23 % at week 24)and class 2 obesity (from 25 % at week 0 to 15 % at week 24).However, obesity class III showed modest change 8 to 6%, (Figure 1, 2).
Figure 1 Gender.
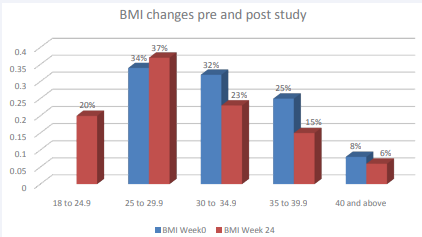
Figure 2 BMI changes pre- and post- study.
On the other hand, 20 % of patient could reach normal BMI by the end of the study. Moreover, percentage of patient with overweight increased from 34% at baseline to 37% at 24 weeks which could be explained by those patients who lost weight and moved from obesity category to overweight category
Glycated hemoglobin (Hb A1c)
The mean HbA1c was measured at 3 periods: week 0, week 12 and week 24. There was significant reduction in HbA1c by 11.58% from 7.12 (SD1.067) at week 0 to 6.28 % (SD1.066) at week 24 of the study, p value <0.05 (Table 4). The net change in HbA1c was -0.84%. This improvement of HbA1c was noticed while medications get reduced/discontinued in most of the patients. The level of control of HbA1c was also analyzed. The percentage of patients with controlled HbA1c (<7%) was increased dramatically from 58% at week 0 to 79% at week 24 and this improvement was noticed while the medications get adjusted. On the other hand, the percentage of patients with uncontrolled Hb A1c (>7%) was reduced from 42 % at the beginning of the study to 21% at the end of the study (Table 3, Figure 3).
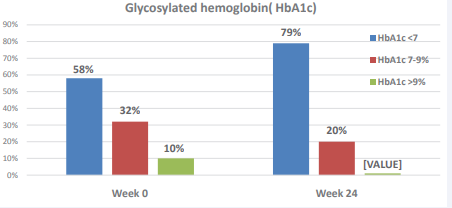
Figure 3 The effect of non- caloric restricted low-carbohydrate diet on Glycosylated hemoglobin (HbA1c).
In linear regression analysis, there was no absolute correction between HbA1c, body weight and waist circumference.
Other metabolic effect
Regarding the other metabolic measurements, serum triacylglyceride (TAG) showed mean reduction of 21.8% (from 1.47+/- 0.934 at baseline to week to 1.22+/-564 at 24 weeks, p value <0.005. HDL-cholesterol showed steady increase from 1.30 (SD0.297) to 1.40 (SD0.468), p value <0.05. However, the serum total cholesterol and LDL-cholesterol showed insignificant changes throughout the study period (Table 4). EGFR had reduced significantly from 86.73+/- 6.21to 84.92+/-8.47at the end of the study (p value <0.05). No significant changes have been noticed in uric acid, urine ACR and TSH throughout period of the study
Medication adjustment
Diabetic medications adjustment was also studied. At baseline there was 44(62%) patients on monotherapy, 25(35.2%) patients on dual therapy and 2(2.8%) patients were on triple OHA. Post study, there was 33(46.5 %) patients on monotherapy,17 (23.9%) patient on dual therapy, 3 (4.2%) patients were on triple therapy and 18(25.4 %) patients were able to quite all oral hypoglycemic medications (Figure 4, Table 5).
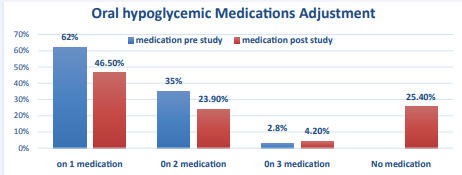
Figure 4 Oral hypoglycemic Medications Adjustment.
Table 5: Oral hypoglycemic medication adjustment.
| Medication adjustment | medication post study | Total | ||||
| No medication | on 1 medication | 0n 2 medication | 0n 3 medication | |||
| medication pre study | 1 medication | 17 (23.90%) | 26 (36.60%) | 1 (1.40%) | 0 | 44 (62%) |
| 2 medication | 1 (1.40%) | 7 (9.9%) | 16 (22.5%) | 1 (1.4%) | 25 (35.2%) | |
| 3 medication | 0 | 0 | 0 | 2 (2.8%) | 2 (2.8%) | |
| Total | 18 (25.4%) | 33 (46.5%) | 17 (23.9) | 3 (4.2) | 71 | |
Out of 44 patients who were on monotherapy, 17 patients had discontinued the Oral hypoglycemic medications, 26 had continued same dose of OHA, and one patient shifted to dual therapy at end of the study (Table 5). On the other hand, out of 25 patients who were on dual therapy, only one patient could quite his medication, 7 patients had reduced their medications to one drug, 16 patients continued same dose and1 patient increased to three medications. None of the patient who was in triple oral hypoglycemic medications could manage to adjust their doses yet their HbA1c had improved over 24-week period. Overall 25.4 % of the patient were able to quite the medication, 9.9 % could able to decrease the dosage,2.8 % increased and 62 % continued same dose (Table 6).
Table 6: Summary of oral hypoglycemic medication adjustment.
| Row Labels | Count of Medication adjustment |
| Increased | 2.8% |
| Decreased | 9.9% |
| Stopped | 25.4% |
| Same | 62.0% |
Throughout the study, the dyslipidemia and hypertension medications were not adjusted.
Adverse effect
The main side effect of this diet was headache accompanied by constipation especially in the first week of the study. Fiftyfour percent of patients reported headache and 66 % reported constipation. However, the intensity of this side effect fades up with continuation of the diet regimen. None of the participant had symptoms of hypoglycemia or fatigability.
DISCUSSION
This is single arm 24-week intervention study which measured the effect of non-caloric restricted low-carbohydrate diet in reversing type 2 DM. Low carbohydrate diet showed significant improvement in glycated hemoglobin, lipid profile, weight and waist circumference and need for diabetic medications over 24-week period. Additionally, this improvement was noticed while diabetic medications get adjusted. Eighteen participants (25%) have successfully managed to quite their medication and majority of them were from monotherapy group. However, this adjustment was done under medical supervision and started as soon as 2 weeks after implementing diet program. Thus, it is recommended that patients with type 2 diabetes mellitus should consult their physician before starting this type of diet regimen as this might need further monitoring of blood sugar level and adjustment of diabetic medications. Overall, this study was of short period and thus the effect of this diet on long term manner yet to be further studied.
Effect of LCD on glycemic control
This study used HbA1c and FBS as indicators for glycemic control. The net reduction in HbA1c was 11.58 % throughout 24- week period, p value <0.05, and this reduction was observed while diabetic medications get adjusted. The possible cause for this improvement was reduction in high glycemic index food and total amount of food rich in carbohydrate without restricting calories intake. It is also shown that this diet contributes to improvement of HbA1c irrespective of weight changes. A study conducted by Yamada et al and his colleagues showed that HbA1c levels were significantly decreased by 7.9% which was lesser than net change achieved in HbA1c in our study [9]. Many other studies have shown similar result comparable to our dietary regimen [1-3,10]. Furthermore, the percentage of people with controlled glycated hemoglobin (HbA1c<7%) had improved dramatically from 58 % at week 0 to 79 % at week 24weeks. This means that regardless of medication adjustment this diet regimen showed tremendous improvement in HbA1c despite the short duration of the study
Medications adjustment
In previous researches, few studies mentioned in detail about medication adjustment throughout study period [5,6]. Other study also focused on adjustment/discontinuation of diuretic medication as LCD regimen might case additional diuresis [5]. This study highlights the oral hypoglycemic medications used before starting of the study and what changed have been made through the 24-week period without making any adjustment in other medication( including diuretics). Most of the medication adjustment were occurred in the monotherapy group where 17 patients have managed to discontinue treatment at the end of the study. However only one patient from dual therapy group could manage to discontinue his medication. None of the patients who were on triple antidiabetic medication could manage to adjust their medication yet their HbA1c showed improvement throughout the study. The possible reason for that is people on dual and triple oral hypoglycemic medications had HbA1c of more than 7 % which need careful monitoring of his/her blood sugar level as well as HbA1c before attempting to reduce the dosage of medication. Additionally, it needs sustainability of diet regimen throughout longer period to get desired effect.ww
Weight reduction
The mean reduction of weight in this study was 7 kg. With compare to other studies, the average reduction was even higher than this study [6,5,11]. The main reason for this difference is the amount of carbohydrate put in each regimen. The lower the carbohydrate diet regimen, the more pronounce is the weight reduction. Other factors might contribute to this variation in net weight reduction are short period of the study, some patient on gliclazide that might adversely increase weight, adherence to physical activity and presence of other comorbidity that interfere with their adherence to physical exercise.
Other metabolic parameters
Similar to previous studies, LCD showed increase in HDLcholesterol, reduction in TAG, but no significant changed was noticed in total cholesterol and LDL-cholesterol [5,6]. On the other hand, previous studies showed no significant effect of LCD on renal function test [5-7]. However, this study showed significant reduction in eGFR over 24-week period (from 86.73+/- 6.21to 84.92+/-8.47, p value <0.05). No significant changes have been noticed in ACR. This change in eGFR might be due to higher amount of protein intake in this type of diet which can adversely affect kidney function over short term period. However, data are still lacking regarding its effect over long term period. Thus, this type of diet might not be a good option for patient with chronic kidney disease (CKD) [12-14]. Further studies are needed to evaluate its effect on patients with normal renal function over longer period.
LIMITATION
The main limitation of this study was its smaller sample size. Other limitations were shorter duration of the study and lack of control group. Furthermore, there might be measurement differences that exist in FBG which were measured at home by the patients themselves using different calipered glucometer. Finally, there was no upper limited sit for exercise the patient might do in daily bases.
CONCLUSION
Non-caloric restricted low- carbohydrate diet showed significant improvement on glycemic control, HDL- cholesterol, and TAG, weight and waist circumference despite the short duration of the study. Additionally, there was significant improvement in term of drug reduction/discontinuation. However, there is concern that this diet might adversely affect kidney function over short period of time which was not shown in previous studies. Its effect over long period of time is yet to be studied. Further studies with control group are recommended to address the effectiveness of this diet and compare it with conventional diet used for type 2 DM.
AUTHOR CONTRIBUTIONS
I would like to give my thanks and gratitude to Mrs. Sumaya Ambosaidi, dietitian at North Mawaleh health center, for her expert contribution in formulating non-caloric restricted lowcarbohydrate diet. I am also thankful and grateful for the patients for their participation in this study as well as SPSS data analysis group for their expert input in analyzing the data.









































































































































