De Novo Hepatocellular Carcinoma Post Liver Transplantation: A Case Report and Brief Review of the Literature
- 1. Department of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh Medical Center, USA
- 2. Department of Internal Medicine, University of Pittsburgh Medical Center, USA
Abstract
Development of new HCC post LT in a patient with no prior history is termed “denovo HCC” and is rare with only thirteen previously reported cases. We describe a patient with Hepatitis C related cirrhosis without HCC prior to transplant, who developed de novo HCC four years after LT and successfully treated with radiofrequency ablation. Given its rarity, no current guidelines exist for surveillance of de novo HCC.
Keywords
Hepatocellular carcinoma; Post liver transplantation ; Immunosuppression.
CITATION
Das R, Lu AD, Malik SM (2016) De Novo Hepatocellular Carcinoma Post Liver Transplantation: A Case Report and Brief Review of the Literature. J Liver Clin Res 3(1): 1021.
ABBREVIATIONS
HCC: Hepatocellular Carcinoma; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; LT: Liver Transplantation; CT: Computed Tomography; MELD: Model for End Stage Liver Disease; IS: Immunosuppression; FNA: Fine Needle Aspiration; NASH: Nonalcoholic Steatohepatitis
INTRODUCTION
Hepatocellular carcinoma (HCC) is a well-known complication of cirrhosis, occurring at a 3-4% annual rate in such patients [1]. The main risk factors for the development of HCC include chronic hepatitis B virus (HBV)infection, chronic hepatitis C virus (HCV) infection, hereditary hemochromatosis, and cirrhosis of any cause [2] in particular alcohol and non-alcoholic fatty liver disease. Liver transplantation (LT) remains the most effective treatment for HCC. When certain criteria are met, specifically no evidence of extra hepatic spread, tumor size and number (single lesion ≤5 cm, up to three lesions none larger than 3 cm) and the absence of vascular or lymphatic invasion, recurrence of HCC can be minimized to 8-9% at 4 years post LT, with an overall recurrence free survival of 83% [3].
The development of HCC post LT in a patient without a history of HCC, either identified on pre-transplant imaging or on the examination of the explanted liver (incidental HCC), is a rare entity known as “de novo HCC.” Per review of the literature, only 14 such cases have been previously reported [4-14]. We report another case of de novo HCC in a cirrhotic allograft within the context of recurrent HCV.
CASE REPORT
er transplant for end-stage liver disease related to genotype1a HCV. Prior to transplantation, the patient had multiple complications, including variceal bleeding, ascites, hepatic hydrothorax, and hepatic encephalopathy. He was initially diagnosed with chronic HCV 5 years pre-transplantation, and had received treatment with combination pegylated interferon and ribavarin, but was unable to achieve a sustained virologic response. Other medical problems include insulin dependent diabetes, psoriasis, and hypertrophic cardiomyopathy, for which he received a myocardial ablation prior to transplant.
A contrast-enhanced computed tomography (CT) performed a few weeks prior to LT showed a liver with cirrhotic morphology containing a tiny hepatic cyst, but otherwise no focal hyper vascular lesions. At the time of transplantation, the patient’s model for end stage liver disease (MELD) score was 36; HCV viral load was 6 million IU/mL.The patient’s donor was a 49 year old woman who was declared brain dead following a severe cerebrovascular accident. The donor had no prior history of liver disease, and her liver was grossly unremarkable.
The explanted liver weighed 1279g, and on selected specimens, had evidence of mildly active hepatitis C (modified histology activity index score of 5/18) and macro and micronodular cirrhosis. Four macro regenerative nodules were also noted on the specimen, up to 1.3 cm in the greatest dimension, with low-grade dysplasia.
Postoperatively, the patient had a complicated hospital course. Immunosuppression (IS) was complicated by marked neurologic side effects with tacrolimus, and ultimately was switched tocyclosporine. He developed renal failure secondary to acute tubular necrosis related to pneumonia and sepsis, eventually requiring dialysis. Finally, he also developed an intraabdominal biloma as a result of a bile leak, requiring exploratory laparotomy and biliary anastomotic repair.
After his initial prolonged postoperative hospitalization, treatment for HCV was attempted on two occasions. Prior to his initial treatment, his viral load peaked at nearly 32 million IU/ mL. On both occasions, he was treated with pegylated interferon alpha-2a (180 υg per week) and ribavarin (400 mg per week). The patient did have a ‘partial’ response with a greater than 2 log drop in viral load, but treatment was prematurely discontinued due to side effects and/or recurrent hospitalizations for various issues.
Six months post LT, liver chemistries showed a total bilirubin of 2.4, AST of 40, ALT of 42, alkaline phosphatase of 172 and gamma-GTP of 26. A liver biopsy showed biliary ductular proliferation, with cholangiolitis and cholestasis and afibrosis score of 5/6. An EGD during this time did show evidence of portal gastropathy, but no evidence of esophageal or gastric varices.
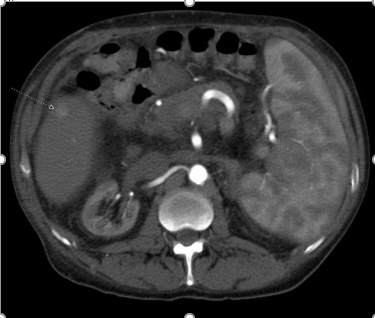
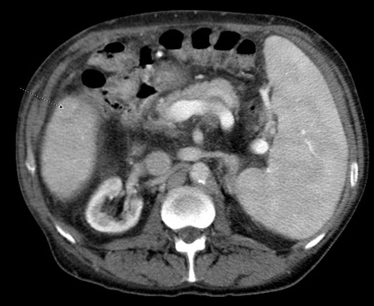
Nearly four years post LT, a CT of the abdomen showed a cirrhotic appearing liver, and a new, 1.4 cm hypervascular lesions in segment 5 with central washout, highly suspicious for HCC (Figures 1,2).
Figure 1: Arterial Phase Contrast Computed Tomography of the Abdomen, showing a 1.4cm hypervascular lesion in segment 5 of the liver.
Figure 2: Portal Venous Phase Contrast Computed Tomography of the Abdomen, showing the same lesion in Figure 1 with washout, diagnostic of hepatocellular carcinoma.
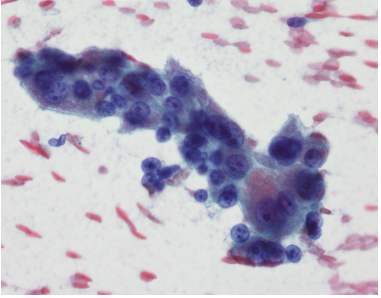
At that time, his laboratory data showed relatively preserved synthetic function, with albumin of 3.5 g/ dL, total bilirubin of 1.2 mg/dL, and INR of 1.2; AFP was 1.5 ng/ mL. A fine needle aspiration (FNA) of the lesion confirmed the diagnosis of HCC (Figure 3).
Figure 3: Cytology of lesion after fine needle aspiration; showing sheets of abnormal hepatocytes with crowding, high nuclear/cytoplasm ratio and nuclear atypia (enlargement, prominent nucleoli), all consistent with hepatocellular carcinoma.
The patient’s HCC was treated with radiofrequency ablation.
Currently, the patient is doing well. He has had no sequelae of portal HTN. Heremains on peritoneal dialysis for management of end-stage renal disease andconsideration is being made for renal transplantation. Repeated surveillance imaging of his liver (now 3 years since his HCC diagnosis) has shown no evidence of recurrent HCC.
DISCUSSION
De novo malignancy is a well-known complication after solid organ transplantation, with non-melanoma skin cancer being the most common. Among patients undergoing LT in particular, the risk seems to be highest among patients with primary sclerosing cholangitis and alcoholic-related liver disease, with nearly a 18-20% probability of developing some type of malignancy at 10 years, with the most common being skin cancer and posttransplant lymphoproliferative disease [15]. Age, cumulative time post LT, smoking, HCV infection and immunosuppression all seem to be independent risk factors in the development of de novo malignancy [16]. De novo HCC, however, remains a very rarely described entity. On our review of the literature (Table 1),
|
Table 1: Summary of Previous De Novo HCC Case Reports. |
|||||||||
|
Case |
Patient Sex/Age |
Donor Sex/Age |
Time from OLT to HCC |
Immunosuppression |
HCC Size and Differentiation |
AFP at diagnosis |
Etiology/Presence of Cirrhosis |
HCC Treatment |
Outcomes |
|
Saxena et al. (1999) |
64/M |
65/F |
7 yrs |
prednisone, cyclosporine |
1.5cm well-differentiated |
73.6 |
recurrent HCV cirrhosis |
none--HCC found in 2nd explanted liver |
died of sepsis 75 days after 2nd OLT for decompensated cirrhosis |
|
Levitsky et al. (2002) |
48/M |
(N/A) |
5 yrs |
prednisone, cyclosporine, azathioprine |
6.5cm well-differentiated with satellite nodules |
(N/A) |
recurrent HCV cirrhosis |
none--patient declined |
(N/A) |
|
Flemming et al. (2003) |
M |
M |
9 yrs |
HBV Ig |
1.2cm less well-differentiated lesion |
(N/A) |
recurrent HBV cirrhosis |
surgical resection |
free of disease 2 yrs after resection |
|
Flemming et al. (2003) |
M |
M |
8 yrs |
HBV Ig |
2cm poorly differentiated lesion |
(N/A) |
recurrent HBV cirrhosis |
underwent second tranplant 3 yrs after diagnosis |
free of disease 1 yr after retransplantation |
|
Torbenson et al. (2005) |
51/M |
(N/A) |
8.5 yrs |
(N/A) |
1cm moderately differentiated HCC |
18 |
recurrent HBV cirrhosis |
none--retransplanted for decompensated HBV cirrhosis |
died 1 month after retransplantation secondary to sepsis |
|
Croitoru et al. (2006) |
61/M |
81/F |
6 yrs |
prednisone, cyclosporine |
3 moderate-to well differentiated nodules (0.6, 1.2, and 2.5cm) with many dysplastic nodules throughout |
12.2 |
recurrent HCV cirrhosis |
none--HCC lesions found in 2nd explanted liver |
received 2nd OLT for decompensated graft failure |
|
Sotiropoulos et al. (2006) |
61/F |
(N/A) |
22 yrs |
(N/A) |
6cm locally invading lesion |
(N/A) |
recurrent HCV cirrhosis |
chemoembolization x3 |
died from cardiac complications of MI 23 yrs 11 mo after HCC diagnosis |
|
Sotiropoulos et al. (2006) |
65/M |
(N/A) |
5 yrs |
(N/A) |
5cm primary lesion and 2 satellite lesions |
(N/A) |
recurrent EtOH cirrhosis |
percutaneous RFA |
died from complications of brainstem ischemia 4 yrs after HCC diagnosis |
|
Kita et al (2007) |
43/M |
(N/A) |
14 yrs |
(N/A) |
3cm poorly differentiated lesion without portal vein invasion |
(N/A) |
recurrent HBV cirrhosis |
TACE |
free of HBV and HCC 2 yrs after second transplantation |
|
Vernadakis et al. (2009) |
59/M |
67/M |
3 yrs |
cyclosporine, MMF, prednisone |
4cm moderately differentiated lesion |
2 |
20% steatosis, no cirrhosis |
partial liver graft resection on seg VII |
normal LFTs and no signs of HCC recurrence 12 months after resection |
|
Yu et al. (2013) |
36/M |
(N/A) |
2 yrs |
tacrolimus, MMF, prednisone |
0.7cm nodule with lung lesion, FNA confirmed HCC |
400 |
recurrent HBV |
RFA, radiotherapy for lung metastasis |
died from respiratory failure 4 months after diagnosis |
|
Tamè et al. (2015) |
54/M |
(N/A) |
6 yrs |
tacrolimus, prednisone |
3 nodules (1.8, 1.0, and 0.6cm), FNA with microsatellite analysis confirmed HCC |
(N/A) |
recurrent HCV cirrhosis |
TACE |
awaiting retransplantation |
|
Tamè et al. (2015) |
44/M |
F |
6 yrs |
tacrolimus, prednisone |
3.5cm lesion, FNA confirmed HCC |
N/A |
recurrent cirrhosis due to sclerosing cholangitis |
Sorafenib |
metastatic HCC undergoing oncologic treatment |
|
Saab et al. (2015) |
47/F |
(N/A) |
19 yrs |
tacrolimus, MMF |
4.7x3.9cm hypodense lesion on CT, FNA confirmed HCC |
>1000 |
recurrent HCV cirrhosis |
chemoembolization x3, RFA, sorfenib |
died from respiratory failure and metastatic HCC 2 yrs after retransplantation |
|
Current patient |
41/M |
49/F |
4 yrs |
cyclosporine |
1.4cm hypervascular lesion with central washout on CT, FNA confirmed HCC |
1.5 |
recurrent HCV cirrhosis |
RFA |
no signs of HCC recurrence 3 years after resection |
this has been reported only 14 times previously [4-14]. In seven of these reports, de novo HCC occurred in the setting of recurrent HCV; in the remaining cases, it occured in the setting of either recurrent HBV or alcohol related liver disease, with one case occurring in recurrent sclerosing cholangitis. The time to development to HCC in these cases was highly variable—from 2-22 years—and was detected either by imaging or on explanted liver after repeat transplantation.
Low-grade dysplastic nodules were noted to be present on our patient’s explanted liver, but such lesions have not been associated with the subsequent development of HCC [17]. Interestingly all but two of the reported cases of de novo HCCs occurred in male patients. Patients in these cases were also exposed to varied immunosuppression. The exact role of specific IS on the recurrence of HCC post LT is not well known [18].
Given the rarity of this occurrence, not surprisingly, no guidelines exist at present for surveillance of HCC in patients post liver transplantation, even among those with recurrent allograft cirrhosis. In an era where HCV is soon becoming a curable disease, non-alcoholic steatohepatitis (NASH) is on the verge of becoming the most common indication for liver transplantation. A significant number of patients with NASH cirrhosis will have HCC at the time of LT [19]. However, the natural history of NASH cirrhosis, post-LT, remains relatively unclear [20], but recurrent NASH will almost certainly lead to allograft cirrhosis in a percentage of patients. Will recurrent NASH cirrhosis be a risk factor in the development of de novo HCC? Only time will answer this very important question. Until then it seems reasonable to pursue periodic imaging to screen for HCC post LT, especially in male patients with recurrent HCV and allograft cirrhosis. Early detection and local therapy may avert the need for repeat transplantation and lead to better long term outcomes.
ACKNOWLEDGEMENTS
The authors would like to acknowledge pathologists Gabriela Quiroga-garza, MD, and Robert Peel, MD, for interpreting and providing the cytology images included in the case report.