L-Ornithine L-Aspartate (LOLA) for the Treatment of Hepatic Encephalopathy in Cirrhosis: Evidence for Novel Hepatoprotective Mechanisms
- 1. Department of Medicine, University of Montreal, Canada
- 2. Clinic of Gastroenterology, City Hospital Magdeburg, Germany
Abstract
Evidence suggests that L-ornithine L-aspartate (LOLA) has direct hepatoprotective properties in chronic liver disease. Such evidence includes reports of attenuation of increased liver enzymes and bilirubin as well as improvements in prothrombin times, Child-Pugh and MELD scores in patients with cirrhosis and HE. These improvements in markers of improved liver function occur in parallel with the reduction in circulating ammonia and improvements in hepatic encephalopathy (HE). Hepatoprotective properties of LOLA occur in patients with cirrhosis and overt HE (OHE) minimal HE (MHE) or HE resulting from post transjugular intrahepatic portal-systemic shunt (post-TIPS HE). Several mechanisms have been proposed to account for hepatoprotection due to LOLA. Metabolic changes mediated directly via L-ornithine and/or L-aspartate and related metabolites (glutamate, glutamine, glutathione) as well as increased synthesis of NO via the increased production of arginine are considered to play a role. Anti-oxidant actions may occur due to increased synthesis of glutathione from glutamate and reduced tissue concentrations of potentially harmful reactive species. In addition, improvement of hepatic microcirculation via NO could also contribute to the hepatoprotective properties of LOLA. These findings strongly suggest that the ammonia-lowering properties of LOLA in patients with cirrhosis could result not only from the ability of the constituent amino acids L-ornithine and L-aspartate to stimulate ammonia incorporation into urea and glutamine in residual hepatocytes of the damaged liver. LOLA may also directly limit hepatocyte damage and necrosis. Further studies are now required in order to more closely examine the validity of the LOLA-hepatoprotection hypothesis from the clinical standpoint.
Keywords
Cirrhosis; Hepatic encephalopathy; Ammonia; L-ornithine L-aspartate; Hepatoprotection; Clinical Trials; Liver Function; Bilirubin; Liver enzymes; NCT-A; Psychometric tests.
CITATION
Butterworth RF, Gruengreiff K (2018) L-Ornithine L-Aspartate (LOLA) for the Treatment of Hepatic Encephalopathy in Cirrhosis: Evidence for Novel Hepatoprotec tive Mechanisms. J Liver Clin Res 5(1): 1044.
ABBREVIATIONS
ALT: Alanine Transaminase; AST: Aspartate Aminotrans ferase; GS: Glutamine Synthetase; GSH: Glutathione; HE: Hepatic Encephalopathy; LOLA: L-Ornithine L-Aspartate; MELD: Model of End-Stage Liver Disease; MHE: Minimal Hepatic Encephalopathy; NAFLD: Non-Alcoholic Fatty Liver Disease; NCT: Number Connec tion Test; OHE: Overt Hepatic Encephalopathy; PCA: Portacaval Anastomosis; PT: Prothrombin Time; RCT: Randomised Control led Trial; TIPS: Transjugular Intrahepatic Portosystemic Shunt
INTRODUCTION
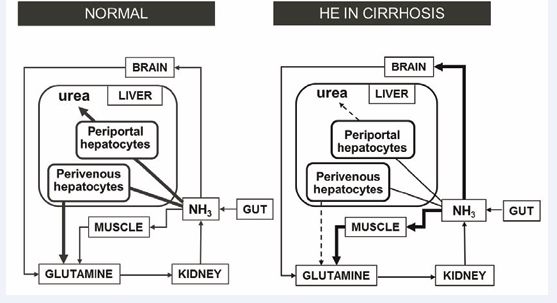
HE in cirrhosis is a serious neuropsychiatric complication that is characterized by alterations of personality, sleep disturbances, motor coordination and cognitive function progressing from inability to compute simple tasks to stupor and coma. A substantial body of evidence supports the notion that increases of circulating ammonia resulting from the cirrhotic liver’s reduced capacity to remove it in the form of urea and/or glutamine is the principal cause of HE in cirrhosis. LOLA is a mixture of endogenous amino acids with the demonstrated capacity to increase ammonia removal by residual hepatocytes and skeletal muscle of patients with cirrhosis (Figure 1) as demonstrated in multiple randomized controlled clinical trials [1-4] as well as in a systematic review and meta-analysis [5].
Figure 1: Inter-organ trafficking of ammonia: effect of cirrhosis. Under normal physiological conditions, gut-derived ammonia gains access to the brain and peripheral organs. Excess ammonia removal occurs principally in the liver via the production of urea and glutamine in periportal and perivenous hepatocytes respectively (6) and also, to a limited extent via glutamine production in muscle. In cirrhosis, hepatic ammonia removal is decreased by up to 80% and muscle takes over the task of ammonia removal as glutamine following induction of the gene coding for GS, the enzyme responsible.
Hepatoprotection by LOLA: Evidence from clinical trials
This area of research followed the publication of a ground breaking report that LOLA has, in addition to its established role as an ammonia scavenger, direct protective effects on the liver per se. The report [6,7] in 314 patients with cirrhosis described the effects of treatment with a range of doses of oral LOLA for periods of from 30-90 days. Severity of fatigue and HE improved significantly with 49% of mild HE patients showing complete recovery of mental state. More importantly, in the context of the present review, plasma concentrations of liver enzymes were significantly attenuated (by up to 70%) in these patients and this was accompanied by comparable reductions of total bilirubin indicative of improved hepatic function (Table 1).
|
Table 1: Effect of LOLA (oral formulation) on liver enzymes and bilirubin in 314 patients with cirrhosis (7). |
||||
|
|
ASAT (U/l) |
ALAT (U/l) |
yGT (U/l) |
Bilirubin (mg/dL) |
|
Start of LOLA treatment |
58.0+/-53.9 |
58.8+/-60.0 |
232.7+/-266.8 |
3.11+/-6.85 |
|
End of LOLA treatment |
38.7+/-45.6 |
39.2+/-41.2 |
115.8+/-137.2 |
2.22+/-4.29 |
|
Difference (%) |
-33.33 |
-33.29 |
-50.24 |
-28.3 |
Although observational in nature and uncontrolled, this study represents the first report of a beneficial effect of LOLA on markers of liver function and symptoms of HE in patients with cirrhosis. Similar findings of hepatoprotective properties of LOLA in randomized controlled trials (RCTs) were subsequently reported in patients with a wide range of HE subtypes including OHE, MHE and post TIPS HE [1-4].
OHE
This clinically apparent type of HE is generally diagnosed according to the severity of neurological symptoms using established Westhaven criteria. A study of 85 patients study of 85 patients with cirrhosis and mild-to-severe OHE were treated with intravenous LOLA (20g/d for 7 days) compared to a control group receiving standard comprehensive care. As expected, treatment with LOLA resulted in lowering of blood ammonia and improvement in severity of HE. Increased serum transaminase levels were significantly attenuated and accompanied by reductions in total bilirubin in these patients [4].
Results of a subsequent randomized clinical trial (RCT) in 120 patients with cirrhosis of predominantly non-alcoholic etiology and mild-to-severe OHE revealed that intravenous LOLA (20g/d for 3 days) resulted in decreased serum bilirubin together with a shortening of prothrombin times (PT) compared to placebo treated controls [1]. Duration of hospital stay was significantly shorter in LOLA-treated patients in this study.
In the studies described above, treatment of patients with cirrhosis with intravenous LOLA (20g/d) for up to up to one week was sufficient to result in improvements in mental state and lowering of blood ammonia together with reductions of liver enzymes and bilirubin and improved PT suggesting that improvement of liver function played a significant role. Indeed, multivariate analysis revealed that improvement of PT was an independent factor associated with definitive improvement of mental state in patients with severe OHE (grades II and above) [1].
MHE
Patients with cirrhosis and MHE present with a normal neurological examination and no obvious clinical signs. Alterations of psychomotor speed, subtle changes in attention and executive decision making much of which is diagnosed by established psychometric testing paradigms form the basis for MHE diagnosis. MHE has a profound influence on quality of life and may affect the patient’s ability to drive an automobile [8].
Patients with cirrhosis and MHE were found to benefit from treatment with LOLA with 100% of patients treated with intravenous LOLA (20g/d for 3 days) showing decreased blood ammonia and improvement of number connection test-A (NCT-A) scores compared to a 50% response in placebo-treated controls (p<0.001) [1]. In a later trial assessing the efficacy of the oral formulation of LOLA (5g/d tid for 60 days), 64 patients with cirrhosis and MHE showed improvement in psychometric test scores as well as a slowed progression to OHE six months post-treatment [2]. Moreover, in this trial, the findings of significant improvements in Child-Pugh and Model of end-stage liver disease (MELD) scores in patients receiving LOLA led the authors to conclude that the lowering of circulating ammonia and delayed progression of HE was directly the result of improved liver function.
Post-TIPS HE
The transjugular intrahepatic portosystemic shunt (TIPS) procedure in patients with cirrhosis is a widely-accepted treatment of portal hypertension and thus the prevention of gastrointestinal bleeding. It is also of value for the treatment of ascites in these patients. However, TIPS is associated with an incidence of new or worsening episodes of HE in up to 30% of patients. Following TIPS, splanchnic blood bypasses the liver and gains access to the systemic circulation. Both venous and arterial ammonia concentrations are increased post-TIPS and the degree of hyperammonemia post-TIPS is a predictor of HE grade [9].
A report of an RCT of 40 patients with cirrhosis who received successful TIPS described the effects of treatment with intravenous LOLA (30g/d for 7 days) or control infusions [3]. Changes in both fasting and post-prandial ammonia concentrations significantly favoured the LOLA group and patients in this group had significantly greater improvements of mental state assessed by psychometric testing on days 1, 4 and 7 post-TIPS. A significant lowering of blood transaminases and bilirubin together with stabilization of MELD scores were also reported in LOLA-treated but not control patients leading the authors to suggest that the prophylactic use of LOLA for one week may be sufficient to alleviate the hepatocellular dysfunction and damage induced by TIPS. A lower incidence of progression from MHE to OHE was noted in the LOLA treatment group.
Hepatoprotection by LOLA: mechanistic studies
Mechanisms purported to be responsible for the protective properties of LOLA relate to a range of actions of the agent’s constituent amino acids, L-ornithine and L-aspartate. Such actions appear to be mediated via glutamine production, antioxidants and/or improved hepatic microcirculation as summarized below.
Actions of LOLA on liver biochemistry and metabolism
Ammonia removal by the liver relies on two independent metabolic pathways namely urea synthesis and the synthesis of glutamine (Figure 1). Ammonia detoxification is impaired by up to 80% in patients with cirrhosis. The efficacy of LOLA is based on urea synthesis in the residual 20% of hepatocytes together with ammonia incorporation into the glutamine molecule in skeletal muscle [10]. In a study of the effects of intravenous infusions of LOLA on plasma ammonia and metabolically-related amino acids in patients with cirrhosis, it was demonstrated that defective urea production was significantly increased [11]. This finding is consistent with a previous report that, in isolated hepatocytes, urea synthesis from ammonia is limited by the availability of L-ornithine [12].
L-aspartate is metabolized in intestinal mucosal cells by transamination to alanine and oxalacetate and exposure to alanine has been shown to lead to reduced enzyme release from normal hepatocytes or those injured by the hepatotoxin D-galactosamine resulting in attenuation of increased plasma liver enzymes. Treatment of patients with cirrhosis by infusions of LOLA results in a significant 2-fold increase of plasma alanine [11].
Role of glutamine
Patients with cirrhosis treated with LOLA manifest significant increases of plasma glutamate and glutamine [11] (Table 2).
|
Table 2: Blood concentrations of ammonia and amino acids before and after 8h infusions of 40g LOLA in patients with cirrhosis (11). |
||||
|
|
Before Treatment |
After Treatment |
Difference (%) |
|
|
Ammonia |
placebo |
212+/-20 |
252+/-20 |
+40 |
|
LOLA |
196+/-6 |
208+/-21 |
+12* |
|
|
Glutamate |
placebo |
34+/-3 |
26+/-3 |
+2 |
|
LOLA |
34+/-3 |
72+/-15 |
+39** |
|
|
Glutamine |
placebo |
855+/-49 |
1022+/-72 |
+167 |
|
LOLA |
882+/-76 |
1092+/-70 |
+209 |
|
|
Alanine |
placebo |
344+/-19 |
491 +/-26 |
+147* |
|
LOLA |
341+/-31 |
597+/-32 |
+256** |
|
|
Arginine |
placebo |
121+/-15 |
124+/-14 |
+3 |
|
LOLA |
97+/-14 |
126+/-9 |
+29* |
|
|
*p<0.05 compared to before treatment **p<0.05 compared to placebo |
||||
Glutamine synthesis from LOLA results from a 2-step reaction involving the transamination of L-ornithine to glutamate which is the obligate substrate for the enzyme glutamine synthetase (GS) [10] (Figure 1). Increased glutamine synthesis in LOLA treated patients occurs in residual perivenous hepatocytes as well as in skeletal muscle; induction of the mechanism involving liver and muscle occurs as a result of a post-translational up regulation of the GS gene [13]. Increased synthesis of glutamine represents not only one of the key steps in the scavenging of excess ammonia but may also play an important step implicated in the hepatoprotective properties of LOLA.
A recent upsurge in interest focuses on glutamine’s role in anti-oxidant pathways. For example in an experimental model of non-alcoholic fatty liver disease (NAFLD), a disorder situated along the continuum of liver damage from simple steatosis to cirrhosis and currently the most common chronic liver disease in Europe, oral glutamine supplementation is hepatoprotective [14,15]. These beneficial effects of glutamine in these experimental situations probably result from increased formation of the anti oxidant glutathione (GSH) as discussed below.
Role of glutathione
In studies of HE resulting from liver injury due to exposure to the hepatotoxin thioacetamide, treatment with LOLA results in significant decreases of serum levels of liver enzymes (AST, ALT) and bilirubin in addition to significant attenuation of liver cell membrane disruption and tissue necrosis. LOLA treatment also leads to decreased liver tissue concentrations of thiobarbituric acid reactive species, an agent known to cause hepatocellular damage and, importantly, LOLA treatment also resulted in concomitant reversal of the decreases in concentration of the anti-oxidant glutathione GSH [16]. Taken together, these findings strongly support an anti-oxidant mechanism of action of LOLA in the hepatoprotection observed in liver failure.
Role of L-arginine/nitric oxide
An alternative mechanism implicated in the hepatoprotective mechanism due to LOLA involves the generation of nitric oxide (NO) from L-arginine. Studies in LOLA-treated experimental HE [10] and in patients with cirrhosis receiving LOLA [11] consistently reveal increases of circulating L-arginine (Table 2) generated from L-ornithine (Figure 1). L-arginine is the obligate substrate for nitric oxide synthase, the enzyme responsible for the synthesis of NO. Administration of L-arginine has been shown to contribute to the improved hepato-vascular perfusion in experimental chronic liver disease by increasing the production of NO [17]. Similar mechanisms are likely to occur in patients with cirrhosis treated with LOLA.
SUMMARY
An increasing body of evidence suggests that LOLA has direct hepatoprotective properties in chronic liver disease. Evidence from uncontrolled clinical studies as well as RCTs includes reports of attenuation of increases of liver enzymes and bilirubin as well as improvements in prothrombin times, Child-Pugh and MELD scores in patients with cirrhosis and HE.
These improvements in indices of liver function occur in parallel with the reduction in circulating ammonia and improvements in encephalopathy grade. These hepatoprotective properties of LOLA occur in patients with cirrhosis and overt HE, minimal HE or post-TIPS HE. Based upon studies in experimental models as well as in patients with cirrhosis and HE, several mechanisms have been proposed to account for these hepatoprotective properties of LOLA. Such mechanisms include metabolic changes mediated directly via L-ornithine and/or L-aspartate and one or more of their active metabolites that include L-glutamine, L-alanine and GSH as well as increased synthesis of NO via the increased production of L-arginine (Figure 1).
Anti-oxidant actions may occur due to increased synthesis of GSH from glutamate generated via transamination of the constituent amino acids of LOLA and reduced tissue concentrations of potentially harmful reactive species. In addition, increased synthesis of L-arginine resulting in increased NO production and improvements of hepatic microperfusion could contribute further to these hepatoprotective properties of LOLA (Figure 1). These findings strongly suggest that the ammonia-lowering properties of LOLA in patients with cirrhosis result not only from the potential of the constituent amino acids (L-ornithine and L-aspartate) to stimulate ammonia incorporation into urea and glutamine in residual hepatocytes of the damaged liver. LOLA also directly limits hepatocyte damage via mechanisms involving increased glutamine, the antioxidant GSH and the L-arginine/NO system. Further studies together with appropriately-controlled clinical trials are now required in order to more closely examine the validity of the LOLA-hepatoprotection hypothesis from a clinical standpoint.