Role of Liv.52 DS Tablets as a Hepatoprotective Agent in Tuberculosis Patients Receiving Antitubercular Drugs: A Double Blind Placebo Controlled Study
- 1. Otoch Manramba Medical Institute, Ulaan Baatar, Mongolia
- 2. Songino khairkhan district hospital, Mongolia
- 3. Bayangol district hospital, Mongolia
- 4. Nalaikh district hospital, Mongolia
- 5. Baga nuur district hospital, Mongolia
- 6. Dornogovi provincial hospital, Mongolia
- 7. Bayanzyrkh district hospital, Mongolia
- 8. Department of Clinical trial and Medical Services, The Himalaya Drug Company, India
Abstract
Drug-induced hepatotoxicity is a potentially serious adverse effect of antituberculosis treatment regimens containing Isoniazid, Rifampicin and Pyrazinamide short-course (DOTS) strategy for control of tuberculosis endorsed by the World Health Organization (WHO) and all the three drugs have been observed to have hepatotoxic potential. A review of available literature suggests that reduction in lipid peroxide content in tissue and increase in superoxide dismutase, catalase, glutathione, and glutathione-s-transferase and glutathione peroxidase activities should help to maintain liver cell integrity and control the increase in level of liver enzymes. Liv.52 DS, a polyherbal formulation, has been used and clinically documented extensively for its various benefits in the management of various hepatic disorders over the past 55 years. In the present study, Liv.52 DS was evaluated in 90 cases of TB-DILI for hepatoprotective activity in a double blind placebo controlled design for 6 months. Statistical analysis showed improvement in haemoglobin, serum protein and the signs and symptoms of TB-DILI. Also, significant reduction was observed in liver enzymes; SGOT, SGPT, Serum Alkaline Phosphatase and serum bilirubin. Thus, the results were suggestive of positive outcome in the management of TB-DILI by Liv.52 DS formulation.
Keywords
TB-DILI ; Liv 52 DS ; Hepatoprotective.
CITATION
Choijamts G, Narantstsetseg, Oyunerdene, Zagirjav, Erdenesuvd, et al. (2018) Role of Liv.52 DS Tablets as a Hepatoprotective Agent in Tuberculosis Patients Receiving Antitubercular Drugs: A Double Blind Placebo Controlled Study. J Liver Clin Res 5(1): 1042.
ABBREVIATIONS
WHO: World Health Organization; TB-DILI: Antituberculosis Drug-Induced Liver Injury; DILI: Drug-Induced Liver Injury
INTRODUCTION
Tuberculosis is a common problem in India and worldwide, especially after the recent increase in incidence of acquired immunodeficiency syndrome (AIDS) as well as Multiple Drug Resistant Tuberculosis (MDR-TB) due to inefficient management [1]. Each year an estimated eight million new cases and two million deaths occur due to TB world wide [2].
Mongolia is one of the seven TB high burden countries in the WHO Western Pacific Region. In 2011, there were 3985 TB cases in Mongolia. Among them 1,723 cases were smear positive pulmonary cases. Mongolia achieved the regional WHO targets for finding and curing TB. Currently, case detection and treatment success rates have reached 72% and 84% respectively. According to WHO, TB prevalence was 331 per 100 000 population and mortality rate was 5 per 100 000 population in 2010 in Mongolia. Mongolia (population: 2.7 million) ranks fourth among the high tuberculosis (TB) burden countries in the World Health Organization (WHO) Western Pacific Region [3].
Drug-induced liver injury (DILI) is a minor but significant cause of liver injury across all regions. Antituberculosis druginduced liver injury (TB DILI) is a leading cause of DILI and druginduced acute liver failure (DIALF). Although TB DILI develops more commonly in males, ALF is noted to be commoner in females with a worse prognosis [4].
The Multidrug-resistant TB (MDR-TB) does not respond to isoniazid and rifampicin, the 2 most powerful anti-TB drugs. The 2 reasons why multidrug resistance continues to emerge and spread are mismanagement of TB treatment and person-to-person transmission. Most people with TB are cured by a strictly followed, 6-month drug regimen that is provided to patients with support and supervision.
At present, most commonly used anti-TB drugs are more or less hepatotoxic, especially when several anti-TB drugs are used in combination. Liver dysfunction caused by anti-TB drugs often results in interruption of anti-TB therapy and acute hepatic failure [5,6]. Drug-induced hepatotoxicity is a potentially serious adverse effect of antituberculosis treatment containing isoniazid, rifampicin and Pyrazinamide [7] in short-course treatment (Directly observed therapy -DOTS) for control of tuberculosis endorsed by the World Health Organization (WHO) and all the three drugs have been observed to have hepatotoxic potential [8].
DILI may result from direct toxicity of the primary compound, a metabolite, or from an immunologically mediated response, affecting hepatocytes, biliary epithelial cells, and/ or liver vasculature. In many cases, the exact mechanism and factors contributing to liver toxicity remain poorly understood. Predictable DILI is generally characterized by certain doserelated injury in experimental animal models. Injurious free radicals cause hepatocyte necrosis in zones farthest from the hepatic arterioles, where metabolism is greatest and antioxidant detoxifying capacity is the least [8].
The pathogenesis of hepatotoxicity is not entirely clear, but INH and RMP induced damage may involve oxidative stress, lipid peroxidation, choline deficiency leading to lowering of phospholipids protein synthesis with alteration in cell wall configuration, reduced glutathione level and activation of CYP2E1 [9]. It is well known that some non-toxic herbs are having opposite activities in the form of membrane stabilizing, anti-oxidative and CYP2E1 inhibitory effects. A review of available literature suggests that reduction in lipid peroxide content in tissue and increase in superoxide dismutase, catalase, glutathione, glutathione-s-transferase and glutathione peroxidase activities should help to maintain liver cell integrity and control the increase in level of liver enzymes.
Commonly used anti-tubercular drugs, such as isoniazid, rifampicin, pyrazinamide etc., are hepatotoxic [6]. Isoniazid causes hepatic damage either by the toxicity of or hypersensitivity induced by its metabolite-acehydrazide. Rifampicin may accelerate the metabolism of isoniazid as a strong enzyme inducer resulting in the increase of acehydrazide which combines with biomacromolecules in liver leading to hepatocellular damage usually seen in aged patients with excessive drinking, malnutrition or a liver ailment. Pyrazinamide’s hepatotoxicity is dose-dependent and the general dose rarely causes hepatic damage. Isoniazid and rifampicin are the first line anti- TB medicines because of their strong bactericidal effects. However, Rifabutin, Amikacin, ofloxacin, levofloxacin, etc., in the treatment plan have not been reported with obvious hepatotoxicity [10,11].
TYPES OF DILI
A variety of clinical syndromes may be seen with DILI, even with a single drug.
Hepatic adaptation
Exposure to certain drugs may evoke physiologic adaptive responses. The induction of survival genes, including those that regulate antioxidant, anti-inflammatory and antiapoptotic pathways, may attenuate toxin-related injurious responses. Such injury may also stimulate hepatocyte proliferation and protective adaptation. Asymptomatic, transient elevations of SGPT may reflect slight, non progressive injury to hepatocyte mitochondria, cell membranes, or other structures. Such injury rarely leads to inflammation, cell death, or significant histopathologic changes. Certain toxins, such as ethanol, possibly interfere with these adaptive protective responses. Excessive persistence of an adaptive response may, in some instances, render hepatocytes more vulnerable when they are subjected to additional new insults. The induction of hepatic microsomal (cytochrome P450) enzymes, capable of metabolizing the inducing medication, is another form of hepatic adaptation.
Drug-induced acute hepatitis or hepatocellular injury
A transaminase threshold for clinicopathological significant drug induced hepatitis has not been systematically determined for most medications. Patients who take phenytoin often have transaminase elevation up to three times the Upper limit of normal (ULN), but liver biopsies do not reveal significant pathology. However, in patients treated for rheumatoid arthritis with methotrexate, microscopic evidence of liver injury has been found for any transaminase elevation above the ULN. Patients with acute hepatocellular injury may be asymptomatic or may report a prodrome of fever and constitutional symptoms, followed by nausea, vomiting, anorexia, and lethargy. Histopathology may reveal focal hepatic necrosis, with bridging in severe cases. Markedly increased transaminase concentrations followed by jaundice imply severe liver disease with a 10% possibility of fulminant failure, a maxim known as “Hy’s Law,” after the late hepatologist and DILI expert Hyman Zimmerman. Coagulopathy may develop 24 to 36 hours after onset, although this can subsequently resolve. Coagulopathy persisting beyond 4 days is a poor prognostic sign in acetaminophen-related hepatotoxicity.
Nonalcoholic fatty liver disease
Steatosis, or simple fatty liver, is most commonly caused by obesity, insulin resistance, and probably alterations in triglyceride metabolism. Ethanol, steroids and highly active antiretroviral therapy (HAART) are associated with the development and exacerbation of nonalcoholic fatty liver disease. Constitutional symptoms, nausea, vomiting, or abdominal pain are uncommon. Laboratory findings in severe cases include hypoglycemia, increased serum transaminase concentrations, prolonged coagulation times and metabolic acidosis. Most instances of druginduced steatosis are reversible, if the offending agent is stopped. Persistent steatotic injury may progress to steatohepatitis which is characterized histopathologically by hepatic inflammatory and fatty infiltration and subsequently leading to higher risk of cirrhosis.
Granulomatous hepatitis
Granulomata are common, nonspecific findings in liver histology and are potentially related to infectious, inflammatory or neoplastic etiologies. Hypersensitivity reactions to drugs, such as allopurinol, quinidine, sulfonamides and pyrazinamide are common cause of this type of lesion. Patients may have fever, lethargy, myalgias, rash, lymphadenopathy, hepatosplenomegaly with increased serum SGPT concentration and even vasculitis.
Cholestasis
Bland cholestasis, typically reported with estrogen treatment consists of usually reversible, asymptomatic, increased serum alkaline phosphatase and bilirubin concentration, caused by a failure of bilirubin transport. There is a lack of inflammation in liver tissue.
Chemical cofactors for DILI
Ethanol induces cytochrome P450 2E1, which promotes metabolism of ethanol, acetaminophen and others. Ethanol metabolism yields acetaldehyde, which contributes to glutathione depletion, protein conjugation, free radical generation and lipid peroxidation. Chronic ethanol abuse activates hepatic collagenproducing sinusoidal (stellate) cells, potentially contributing to fibrosis. Some medications such as calcium channel blockers may influence cytochrome P450 and metabolism of potentially hepatoxic drugs, such as simvastatin, which may lead to DILI.
Preexisting liver disease
Abnormal baseline transaminases are an independent risk factor for DILI. Patients with HIV and hepatitis C, appear to have increased frequency of antiretroviral medication–related DILI. The severity of DILI may be greater in patients with underlying liver disease, likely reflecting a summation of injuries [12].
The clinical presentation of ATT-associated hepatitis is similar to that of acute viral hepatitis. ATT can cause varied degree of hepatotoxicity from a transitory asymptomatic rise in transaminases to acute liver failure and the frequency of hepatotoxicity in different countries varies widely from 2-39% [13]. The occurrence of drug-induced hepatotoxicity is unpredictable but it is observed that certain patients are at a relatively higher risk than other populations.
Various herbs have been known to possess hepatoprotective property in drug induced hepatitis and is available in wide spread usage in Ayurvedic system of medicine.
Liv.52 DS, a polyherbal formulation, has been used extensively in the management of various hepatic disorders over the past 55 years. The principal herbs used in the preparation of Liv.52 DS include Capparis spinosa, Cichorium intybus, Solanum nigrum, Terminalia arjuna, Cassia occidentalis, Achillea millefolium and Tamarix gallica.
OBJECTIVES OF THE STUDY
To evaluate the safety and efficacy of Liv.52 DS tablets as a hepatoprotective in tuberculosis patients receiving Antitubercular drugs.
SUBJECT SELECTION CRITERIA
Inclusion criteria
Both male and female patients aged between18 years to 60 years under ATT with any of the following: newly diagnosed cases of tuberculosis, patients on ATT presenting with symptoms of hepatotoxicity like anorexia, abdominal discomfort and pain in right hypochondrium, patients on ATT presenting with or without symptoms of hepatotoxicity with elevated levels of liver enzymes ≥ ULN , patients receiving antitubercular drugs which have a potential to cause hepatotoxicity like Isoniazid, Rifampicin, Pyrazinamide in the first 2 months of Intensive regimen (2HRZE phase). Only the subjects who have not participated in a similar investigation in past four weeks and willing to give a written and video informed consent and follow the study protocol were included.
Exclusion criteria
Subjects with severe metabolic disorders, known history or present condition of allergic response to similar pharmaceutical products, its components or ingredients in the test products, subjects with genetic and endocrinal disorders were excluded. Subjects who have used a similar product in the past four weeks and pregnant and lactating women were also excluded from the study.
Methodology
Ninety subjects who fulfilled the subject selection criteria were selected. All the subjects were instructed regarding the study procedure, investigations and follow up visits and information regarding the contact person during emergency. The study was conducted as per ICH-GCP guidelines after the ethics committee approval.
This was a double blind placebo controlled 2 arm clinical study in which Liv.52 DS was given to 1 arm and placebo tablets in 2nd arm. Subjects in each arm were advised to take the study drug, at a dose of 2 tablets twice daily as per randomization for a period of 6 months. The subjects were evaluated for the efficacy of the treatments during baseline, month 2, month 4 and month 6 intervals during the study period of 6 months. At the checkup clinical response to the treatment, incidence of any adverse events and subject compliance were assessed.
ADVERSE EVENTS
The incidence and type of adverse events if any reported were also tabulated separately. All adverse events, either reported or observed by subjects, were recorded with information about severity, duration, and action taken regarding the study drug. Relation of adverse events to study medication was predefined as “Unrelated” (a reaction that does not follow a reasonable temporal sequence from the administration of the drug), “Possible” (follows a known response pattern to the suspected drug, but could have been produced by the subject’s clinical state or other modes of therapy administered to the subject), “Probable” (follows a known response pattern to the suspected drug that could not be reasonably explained by the known characteristics of the subject’s clinical state) and “Certain” (occurring in a plausible time relationship to drug administration and which cannot be explained by concurrent disease or other drugs).
STATISTICAL ANALYSIS
Results were analyzed statistically for comparison between the groups using Mann Whitney test and Unpaired t test and within the group analysis using Repeated measures of ANOVA followed by Tukey’s multiple comparisons test, Friedman test followed by Dunn’s multiple comparisons test and Paired t test to find out the statistical significance. Safety parameters were analyzed using Paired t test. Analysis was performed using GraphPad Prism software Version 6.07, San Diego, California, USA.
RESULTS
Demographic data of the study patients is given in Table (1).
|
Table 1: Demographic Data. |
||
|
Liv.52 DS |
Placebo |
|
|
Number of subjects |
47 |
43 |
|
Gender (Male: Female) |
(24:22) |
(22:21) |
|
Age in Years Mean ± SD |
29.4 ± 9.8) |
30.7 ± 9.1 |
|
Marital status (Married: Unmarried) |
(27:19) |
(25:18) |
|
Smoking (Yes: No) |
(10:36) |
(13:29) |
|
Alcohol (Yes: No) |
(8:38) |
(7:35) |
|
The form of Tuberculosis |
||
|
Pulmonary tuberculosis |
28 |
30 |
|
Tuberculosis of other organs |
18 |
13 |
|
Duration of tuberculosis suffering (months) |
3.8 ± 2.3 |
5.1 ± 2.1 |
Safety parameters on hematological and biochemical parameters were assessed within the groups as well as between the groups. Hemoglobin improved from 13.17 ± 1.70 to 13.29 ± 1.21 with a significance of p< 0.004 as compared to Placebo. In placebo group there was a slight fall in haemoglobin levels. All other safety parameters were also found to be within the normal limits in either the groups both pre- and post-treatment (Table 2).
|
Table 2: Evaluation of Safety parameters. |
|||
|
Parameters |
Visits |
Liv.52 DS |
Placebo |
|
Hemoglobin (g/L) |
baseline |
13.17 ± 1.70 |
13.20 ± 1.30 |
|
at end of 6th month |
13.29 ± 1.21 q:p<0.004 |
12.68 ± 1.29 |
|
|
ESR (mm/hour)
|
baseline |
15.35 ± 10.83 |
14.00 ± 7.44 |
|
at end of 6th month |
7.17 ± 4.02 a:p<0.0003 q:p<0.0353 |
9.69 ± 6.05 a:p<0.0004 ns |
|
|
Red blood cells (x1012/L) |
baseline |
4.47 ± 0.87 |
4.91 ± 1.87 |
|
at end of 6th month |
4.68 ± 1.65 |
4.62 ± 1.06 |
|
|
Total white blood cells (x109/L)
|
baseline |
8.82 ± 2.91 |
8.66 ± 2.91 |
|
at end of 6th month |
6.49 ± 1.60 a:p<0.0001 |
6.99 ± 2.01 a:p<0.0002 |
|
|
Thrombocytes (x109/L)
|
baseline |
285.7 ± 96.78 |
272.7 ± 77.15 |
|
at end of 6th month |
242.2 ± 43.69 a:p<0.0018 |
243.5 ± 59.84 a:p<0.0471 |
|
|
Between the group analysis: Statistical test: Unpaired t test a: as compared to baseline: q: as compared to Placebo Within the group analysis: Statistical test: Paired t test Minimum significance level p<0.05; ns: Not Significant The values for Total white blood cells and Thrombocytes though significant cannot be attributed to treatment related effects as the values were within the normal range. |
|||
Liver function tests, as part of efficacy parameters were assessed both within and between the groups and as enumerated in Table (3) and (Figures1-5).
|
Table 3: Evaluation of Liver Function Tests. |
|||
|
Parameters |
Visits |
Liv.52 DS |
Placebo |
|
Total bilirubin ( µmol/L)
|
baseline |
11.1 ± 4.59 |
11.37 ± 4.35 |
|
Month 2 |
11.20 ± 3.72 |
11.96 ± 4.77 |
|
|
Month 4 |
10.98 ± 2.94 |
11.66 ± 4.42 |
|
|
Month 6 |
10.65 ± 2.88 q:p<0.0257 |
12.75 ± 5.27 |
|
|
Serum protein (g/L)
|
baseline |
72.06 ± 16.4 |
73.36 ± 9.38 |
|
Month 2 |
73.54 ± 12.25 |
72.15 ± 8.04 |
|
|
Month 4 |
74.48 ± 12.06 |
75.9 ± 7.05 |
|
|
Month 6 |
75.01 ± 6.04 |
74.19 ± 7.03 |
|
|
Serum alkaline phosphatase (U/L)
|
baseline |
185.7 ± 65.37 |
171.5 ± 70.64 |
|
Month 2 |
180 ± 60.47 |
175.6 ± 54.72 |
|
|
Month 4 |
174.8 ± 52.96 |
166.7 ± 55.89 |
|
|
Month 6
|
147.7 ± 48.09 a:p< 0.0024 q:p< 0.0452 |
171.8 ± 59.14 ns ns |
|
|
SGOT Levels(U/L)
|
baseline |
25.21 ± 11.56 |
23.39 ± 10.32 |
|
Month 2 |
23.61 ± 9.65 q:p< 0.0356 |
29.06 ± 14.15 ns |
|
|
Month 4 |
22.53 ± 7.44 q:p< 0.0447 |
26.83 ± 11.95 ns |
|
|
Month 6 |
21.8 ± 6.21 q:p< 0.0003 |
29.41 ± 11.71 ns |
|
|
SGPT Levels (U/L)
|
baseline |
27.23 ± 17.9 |
29.3 ± 20.27 |
|
Month 2 |
23.46 ± 11.23 q:p<0.0251 |
29.75 ± 13.95 ns |
|
|
Month 4
|
20.9 ± 11.31 q:p<0.0036 a:p<0.007 |
28.79 ± 13.3 ns ns |
|
|
Month 6 |
20.45 ± 9.65 q:p<0.0018 |
28.26 ± 12.78 |
|
|
Between the group analysis: Statistical test: Unpaired t test a: as compared to baseline; q: as compared to Placebo Within the group analysis: Statistical test: Repeated Measure ANOVA followed by Tukey’s multiple comparison test; Minimum significance level p<0.05 |
|||
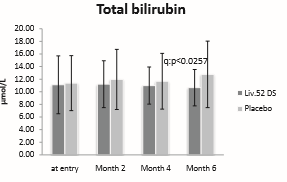
Total bilirubin score baseline was 11.1 ± 4.59 in Liv.52 DS group and 11.37 ± 4.35 in placebo group. At the end of month 6 the total bilirubin score reduced to 10.65 ± 2.88 with a significance of p<0.0257 as compared with placebo group.
Figure 1: Total Bilurubin.
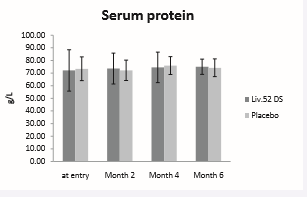
Serum protein showed improvement in both the groups post treatment. Serum protein score at baseline was 72.06 ± 16.4 in Liv.52 DS group and 73.36 ± 9.38 in placebo group. At the end of month 6 the serum protein score was 75.01 ± 6.04 in Liv.52 DS group as compared with placebo group. However the trend towards improvement is comparatively more in Liv.52 DS group as compared to Placebo.
Figure 2: Serum Protein.
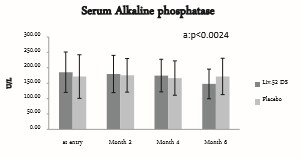
Serum alkaline phosphatase at baseline was 185.7 ± 65.37 in Liv.52 DS group and 171.5 ± 70.64 in placebo group. At the end of month 6, serum alkaline phosphatase was 147.7 ± 48.09 in Liv.52 DS group with a significance of p<0.0452 as compared to placebo group and within the group analysis with a significance of p<0.0024 as compared to baseline in Liv.52 DS group.
Figure 3: Serum Alkaline phosphatase.
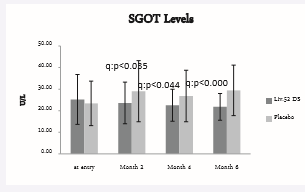
Figure 4 : SGOT levels.
SGOT score at baseline was 25.21 ± 11.56 in Liv.52 DS and 23.39 ± 10.32 in placebo group. At the end of month 2, the SGOT score was 23.61 ± 9.65 with a significance of p<0.0356 as compared with placebo group, which was 29.06 ± 14.15.
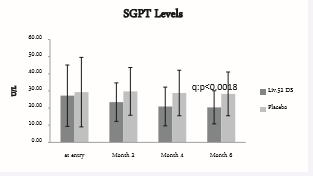
Figure 5: SGPT Levels.
At the end of month 4, the SGOT score was 22.53 ± 7.44 with a significance of p<0.0447 as compared with placebo group which was 26.83 ± 11.95. At the end of month 6, the SGOT score was 21.8 ± 6.21 with a significance of p<0.0003 as compared with placebo group of 29.41 ± 11.71.
SGPT score at baseline was 27.23 ± 17.9 in Liv.52 DS and 29.3 ± 20.27 in placebo group. At the end of month 2, the SGPT score was 23.46 ± 11.23 with a significance of p<0.0251 as compared to placebo group. At the end of month 4, SGPT score was 20.9 ± 11.31 in Liv.52 DS group with a significance of p<0.0036 as compared to placebo group and a significance of p<0.007 as compared to baseline in Liv.52 DS group. At the end of month 6, the SGPT score was 20.45 ± 9.65 with a significance of p<0.0018 as compared with placebo group.
Significant improvement in signs and symptoms of hepatotoxicity due to antitubercular drugs was noted in subjects of both Liv.52 DS group and placebo group. The evaluation of the effect of Liv.52 DS for clinical signs conducted within and between the groups is shown in Table (4).
|
Table 4: Evaluation of Clinical Signs of Antitubercular drugs. |
|||
|
Parameters |
Visits |
Liv.52 DS |
Placebo |
|
Tiredness
|
baseline |
2.59 ± 3.18 |
1.98 ± 2.28 |
|
Month 2
|
1.54 ± 2.23 a:p<0.0129 |
1.28 ± 1.87 a:p<0.012 |
|
|
Month 4
|
0.78 ± 1.49 a:p< 0.0001 |
0.72 ± 1.35 a:p< 0.0001 |
|
|
Month 6
|
0.04 ± 0.29 a:p< 0.0001 q:p<0.0063 |
0.23 ± 0.48 a:p< 0.0001
|
|
|
Fatigue
|
baseline |
2.52 ± 3.22 |
1.93 ± 2.32 |
|
Month 2
|
1.54 ± 2.08
|
1.14 ± 1.7 a:p<0.012 |
|
|
Month 4
|
0.48 ± 1.05 a:p< 0.0001 |
0.47 ± 0.93 a:p< 0.0001 |
|
|
Month 6
|
0.07 ± 0.33 a:p< 0.0001 |
0.19 ± 0.45 a:p< 0.0001 |
|
|
Weight loss
|
baseline |
0.28 ± 0.73 |
0.77 ± 0.92 |
|
Month 2 |
0.14 ± 0.41 |
0.23 ± 0.48 |
|
|
Month 4
|
0 ± 0
|
0.02 ± 0.15 a:p<0.0017 |
|
|
Month 6
|
0 ± 0
|
0.05 ± 0.21 a:p<0.0032 |
|
|
Appetite loss
|
baseline |
1.37 ± 1.82 |
1.14 ± 1.28 |
|
Month 2 |
0.70 ± 1.07 |
0.44 ± 0.77 |
|
|
Month 4
|
0.07 ± 0.33 a:p<0.0008 |
0.16 ± 0.43 a:p<0.0012 |
|
|
Month 6
|
0.02 ± 0.15 a:p<0.0006 |
0.12 ± 0.32 a:p<0.0007 |
|
|
Indigestion
|
baseline |
1.17 ± 1.7 |
0.98 ± 1.41 |
|
Month 2 |
0.54 ± 0.96 |
0.35 ± 0.75 |
|
|
Month 4
|
0.02 ± 0.15 a:p<0.0056 |
0.05 ± 0.21 a:p<0.0104 |
|
|
Month 6
|
0 ± 0 a:p<0.0042 |
0.02 ± 0.15 a:p<0.0058 |
|
|
Within the group analysis; Statistical test: Friedman test followed by Dunn's multiple comparisons test Instruction of filling the table: a: as compared to baseline If positive symptom= 1; Minimum significance level p<0.05 If negative symptom= 0; Between the group analysis If 0-3 symptoms-slight; Statistical test: Mann Whitney test If 3-6 symptoms-average; q: as compared to Placebo; 6-10 symptoms-severe |
|||
Tiredness score at baseline was 2.59 ± 3.18 in Liv.52 DS group and 1.98 ± 2.28 in Placebo. At month 2, tiredness score was 1.54 ± 2.23 with a significance of p<0.0129 as compared to baseline in Liv.52 DS and with a significance of p<0.012 in placebo group. At month 4, it was 0.78 ± 1.49 with a significance of p<0.0001 as compared to baseline score in Liv.52 DS group. In placebo group score was 0.72 ± 1.35 at month 4 as compared to baseline with a significance of p<0.0001. At month 6, tiredness score reduced to 0.04 ± 0.29 with a significance of p<0.0001 as compared to baseline group in Liv.52 DS and in placebo group the score was 0.23 ± 0.48 with a significance of p<0.0001.
Fatigue score at baseline was 2.52 ± 3.22 in Liv.52 DS group and 1.93 ± 2.32 in Placebo. At month 6, fatigue score reduced to 0.07 ± 0.33 with a significance of p<0.0001 as compared to baseline in Liv.52 DS group. In Placebo group score reduced to 0.19 ± 0.45 with a significance of p<0.0001 as compared to baseline values.
Weight loss score at baseline was 0.28 ± 0.73 in Liv.52 DS group and 0.77 ± 0.92 in placebo group. At the end of 4th month and 6th month there was no weight loss in Liv.52 DS group. Where as in Placebo group 0.05 ± 0.21 at month 6, with a significance of p<0.0032 as compared to baseline. In Liv.52 group the weight loss was not observed right from 4th month onwards, whereas in placebo group mild weight loss continued till the end of the treatment.
Score for loss of appetite at baseline in Liv.52 DS group was 1.37 ± 1.82 and 1.14 ± 1.28 in placebo group. At month 2, the score was 0.70 ± 1.07 in Liv.52 DS group and 0.44 ± 0.77 in placebo group. At month 4, appetite loss score further reduced to 0.07 ± 0.33 with a significance of p<0.0008 as compared to baseline. In placebo group, the score reduced to 0.16 ± 0.43 with a significance of p<0.0012 as compared to baseline. At month 6, appetite loss score was 0.02 ± 0.15 in Liv.52 DS group with significance of p< 0.0006 as compared to baseline. Similarly, placebo group score was 0.12 ± 0.32 with a significance of p< 0.0007 as compared to baseline.
Indigestion score at baseline was 1.17 ± 1.7 in Liv.52 DS group and 0.98 ± 1.41 in placebo group. At the end of month 2, indigestion score was 0.54 ± 0.96 in Liv.52 DS group and 0.35 ± 0.75 in placebo group. At month 4, indigestion score further reduced to 0.02 ± 0.15 with a significance of p<0.0056 as compared to baseline and in placebo group score was 0.05 ± 0.21 with a significance of p<0.0104 as compared to baseline. Further at month 6, indigestion was completely relieved. Liv.52 DS group with a significance of p<0.0042 as compared to baseline. In placebo group score reduced to 0.02 ± 0.15 with a significance of p<0.0058 as compared to baseline.
Overall compliance to the study drug was found to be good and there were no adverse effects either reported or observed by the investigator. Improvements in all signs and symptoms were observed Liv.52 DS group.
Survey results assessed by both patient & researcher after the treatment with Liv.52 DS and placebo group are presented in Table (5). Liv.52 DS group showed 5.98 ± 0.15 as compared with placebo group at 5.80 ± 0.52 with a significance of p<0.0357 in Liv.52 DS group.
DISCUSSION
The liver has a central role in drug metabolism and detoxification and is consequently vulnerable to injury. The pathogenesis and types of DILI are presented ranging from hepatic adaptation to hepatocellular injury. Knowledge of the metabolism of anti-TB medications and of the mechanisms of TB DILI is incomplete [12].
The use of multiple regimens, vastly different study populations, varying definitions of hepatotoxicity and different monitoring and reporting practices make it difficult to reach definitive conclusions regarding risks of individual regimens. Overall, the risk of TB DILI in these diverse studies ranges from 5 to as high as 33%. Malnutrition or hypoalbuminemia was also associated with TB DILI in several studies reported from India [12].
The antitubercular drugs have their own hepatotoxic potential but when used in combination with each other, the overall hepatotoxicity may be cumulative. Also, combining these drugs can considerably increase the global risk of hepatotoxicity in the presence of liver disease.
Several types of drug-induced liver damage have been described. These include (i) idiosyncratic damage; (ii) dose dependent toxicity; (iii) induction of hepatic enzymes; (iv) drug induced acute hepatitis; and (v) allergic reactions among others.
Ideally, antituberculosis treatment should be individualized according to the body weight and co-morbid illnesses present in the patient. Whenever feasible, baseline liver function testing must be done. When drug-induced hepatotoxicity is suspected, the patient receiving antituberculosis-treatment should be systematically investigated for other causes such as viral hepatitis and underlying hepatic conditions.
Any possible supportive care to minimize the damage the liver parenchyma will improve the hepatic function in patients receiving Antitubercular treatment and also improves the ATT compliance and overall outcome from tuberculosis. Hence herbal medicines with hepatoprotective potential are required to support in ATT treatment.
Liv.52 DS tablet is a polyherbal formulation, which has been used extensively in the management of liver disorders. In this study the safety and efficacy of Liv.52 DS in preventing hepatic damage due to Antitubercular drugs was investigated. There was significant symptomatic relief from symptoms of liver damage due to antitubercular drugs in Liv.52 DS group. The significant outcome observed in these study patients might be due to the synergistic mechanism of action of all the ingredients of Liv.52 DS.
Hepatoprotective activity of Liv.52 in ATT induced liver injury has been reported previously by multiple authors [14 19]. A meta-analysis of eight clinical studies conducted between 1970 and 1992 in 689 tubercular patients aged 2 months to 60 years receiving antitubercular treatment (ATT) along with Liv.52 or placebo was taken up for this study. Improvement in the various parameters of hepatotoxicity, such as hepatomegaly, anorexia, weight gain, general well-being and liver function test (SGOT), and improvement in the ultrasonographic findings of the hepatobiliary system were taken into consideration. Results of the meta-analysis showed a statistically significant improvement in hepatotoxicity in patients receiving anti-TB drugs and Liv.52. Significant improvements were observed in associated symptoms such as anorexia, weight gain, hepatomegaly and general well-being [19]. Hence, the results from present study are also supported by previous works on Liv.52 formulation.
Liv.52 DS has ingredients like Capparis spinosa, Cichorium intybus, Mandhura bhasma, Solanum nigrum, Terminalia arjuna, Cassia occidentalis, Achillea millefolium and Tamarix gallica and processed in Phyllanthus maderaspatensis, Eclipta alba, Coleus aromaticus, Andrographis paniculata and other hepatoprotective herbs designed for the management of liver disorders. These herbs possess significant hepatoprotective activity and have been used for centuries as a part of the Ayurvedic approach to healthcare. It has a wide spectrum of therapeutic applications from restoring the metabolic efficiency of the liver in various etiological forms of hepatocellular jaundice like infective and chronic active hepatitis to drug-induced hepatitis and alcohol induced hepatic damage. It increases appetite, corrects the hepatitis, cirrhotic conditions and in any hepatotoxic drug regimen. It is also a supportive treatment during hemodialysis and a useful adjuvant with hepatotoxic drugs.
CONCLUSION
Present study indicate good clinical efficacy of Liv.52 DS to prevent the hepatotoxicity due to Antitubercular drugs. There were also significant improvements in liver function parameters. There were improvements in the clinical symptoms and signs of hepatotoxicity. There were no clinically significant short- and long-term adverse events, either reported or observed during the entire study period. Hence, it may be concluded that Liv.52 DS is safe and effective in preventing hepatotoxicity due to Antitubercular drugs.