Adsorption Mechanism of Sodium Metasilicate on Rutile and Almandine Surfaces
- 1. School of Mines and Engineering, Mining and Mineral Processing Engineering Department, Taita Taveta University, Voi, Kenya
- 2. School of Resource and Safety Engineering, Wuhan Institute of Technology, Wuhan, China
- 3. State Key Laboratory of Mineral Processing Science and Technology, Beijing General Research Institute of Mining and Metallurgy, Beijing, China
- 4. School of Mechanical, Manufacturing and Materials Engineering, Mining, Materials and Petroleum Engineering, Department, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya
Abstract
Sodium metasilicate was investigated as a possible depressant in the separation of rutile from almandine. The study determined the floatability of the depressant through micro f lotation tests of single and artificially mixed minerals. The adsorption behavior of sodium met silicate on the rutile and almandine surfaces was determined by the zeta potential measurement and X-ray Photoelectron Spectroscopy (XPS). The flotation tests for both single and artificially mixed minerals demonstrated that Sodium metasilicate could be an efficient depressant in the separation of rutile from almandine. Zeta potential measurements and XPS analysis depicted that more of Sodium metasilicate was adsorbed on almandine surface than that of rutile. Fe ions on the surface of almandine were the main active sites for collector attachment and adsorption. The element Si in sodium metasilicate was postulated to have contributed to depression of almandine too. The adsorption mechanism of sodium metasilicate on the surface of almandine was mainly attributed to the electrostatic interaction as well as chemical bonding.
Keywords
• Sodium Metasilicate
• Rutile
• Almandine
• Adsorption Mechanism
CITATION
Kasomo RM, Kinyua EM, Li H, Mwangi AD, Ombiro S, et al. (2023) Adsorption Mechanism of Sodium Metasilicate on Rutile and Almandine Surfaces. J Materials Applied Sci 4(1): 1006.
INTRODUCTION
Rutile is a very crucial raw material for the extraction of titanium as well as the production of titanium dioxide [1]. Rutile is also employed in the manufacturing of: Titanium sponge, Titanium dioxide pigment, cosmetics pharmaceuticals, plastics, paper and latex rubber [2-6]. Further uses of titanium dioxide may include: military aviation, navigation, machinery, aerospace, seawater desalination and chemical industry among many other uses [7,8]. There are several processes used to upgrade and dress rutile ore which include: magnetic separation, flotation, magnetic flotation, gravity separation and electrostatic separation [9-12]. As aforementioned, these rutile dressing methods sometimes suffer from inadequacies during the concentration process. For instances, there exists very little difference between the densities of rutile and almandine gangue [13].
In regard to this, it is almost impossible to separate the two minerals by use of gravity separation concentration method [14]. Further to this, it is worth mentioning that both rutile and almandine have similar physicochemical characteristics and as such, the successful separation of the two minerals through gravity concentration process might not be attainable [13]. In search for an effective method for separation of rutile from its gangue minerals, a combination of magnetic and froth flotation technique was developed [15]. A challenge is always encountered in this separation technique in that, quite often than not, there is always a substitution of titanium in the rutile crystal lattice occurring by iron in the form of isomorphism, and thus, rutile slimes are often wrapped by almandine-surface, hence making it very difficult for flotation-magnetic separation technique to be effective [13-16]. Froth flotation, has therefore, remained the most reliable and versatile technique in concentration and dressing of the rutile ores [17].
Reagents and their suitable schemes have remained the major challenge in enrichment of ores. Currently the main focus on rutile flotation has been designing the suitable flotation reagents which register rutile of high quality grade. Many studies in the area of rutile flotation have been concentrated on the development, modification and choice of novel collectors to enhance the floatability difference between rutile and its associated gangue so as to enhance its reparability [18-22]. However, several studies have proved beyond reasonable doubts that, no any single collector that possess enough collective as well as selective capabilities, as such depressants studies is of great interest of late. The focus on depressants has been occasioned by the fact that they help the collectors to achieve that most required selectivity as its main function is to render the gangue mineral hydrophilic and hence impairing its flotation as well as aiding selectivity of the collector as much as possible to enhance the grade of the concentrate [11,23].
Sodium metasilicate has remained as one of such depressant in mineral processing operation [24]. It is has proved to be a versatile and non-toxic, as well as a low-cost multifaceted flotation reagent available in many forms and for a wide range of industrial applications [25]. Sodium metasilicate has been commonly utilized in mineral concentrators as a dispersant, rheology modifying, as well as an inorganic depressant whose effectiveness has been reported in many studies [25]. Additionally, sodium metasilicate has found its application in selective separation of hematite from quartz by reverse flotation where promising results were revealed and recorded [26]. Similarly, it was used as a depressor while sodium operate was on the other hand employed as a collector in the flotation separation of fine fluorite from fine quartz. The results revealed that sodium metasilicate helped to depress the hetero-coagulation and therefore improved the separation efficiency in the beneficiation of mixed binary minerals [27]. It is further utilized as an effective depressant in direct flotation of apatite from siliceous phosphate ore using fatty acids and promising results were posted as well [28]. Additionally, it can be used as an activator because it helps in the reduction or elimination of the deleterious effects of slimes on flotation [29]. Furthermore, sodium metasilicate has been used in grinding unit operation as it helps in lowering the viscosity and provides finer grinding or increases throughput for the same particle sizes [30].
Several other studies have been conducted to investigate the effect of sodium metasilicate as a possible dispersant as well as a depressant with different mineral ores whose response and best results have been positively recommended [31-33]. However, despite the above mentioned studies, there is no reliable literature and information on the proper mechanism of how sodium metasilicate depresses iron-bearing silicate and its related minerals. For instance, the depressing action and mechanism of sodium metasilicate on the surface of iron containing silicate has not been systematically explained before and no enough data on the same which has been reported so far.
In this current study, the flotation behavior of rutile and almandine employing sodium metasilicate as a depressant, octadecylamine, polyoxyethylene ether (AC1815) as the collector is investigated in details is using micro-flotation tests for single as well as the artificially mixed minerals. The adsorption phenomenon of sodium Metasilicate on the almandine and rutile surfaces was clearly unraveled by use of contact angle and zeta potential measurements as well as XPS analyses. The limitation of this study will be the fact that in rutile gangue minerals the authors concentrated with almandine only and did not conduct any comparisons studies with other gangue minerals like hornblende among others.
MATERIALS AND METHODS
Materials
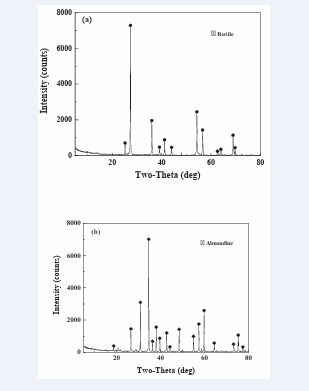
The representative sample of rutile for this research work was obtained from Hainan province, China. It was a tabling concentrate, with 98 wt% TiO2 contents. The sample preparation included grinding it in a porcelain ball mill and subsequently screened it by a wet sieve to obtain the final size fraction of -150 +58 μm suitable for micro flotation and other tests. Almandine sample on the other side was a magnetic concentrate procured from Donghai country, Jiangsu province in China which had 97 wt% purity. Its preparation entailed the dry magnetic separation and then vibration mill. Finally, the ground sample was screened to obtain a size fraction of –150 +58 μm. The bulk constituents of the samples were analyzed by chemical analysis methods, where X-ray fluorescence spectrometry (XRF) test was conducted and the results are depicted in (Table 1). Additionally, X-ray diffraction (XRD) was used to characterize the mineral compositions. The results can be found in (Figure 1a,b) respectively.
Figure 1: XRD pattern of a. Rutile; b. Almandine.
Reagents
Octadecylaminepolyoxyethylene ether (AC1815), which had 99wt% purity, was obtained from Haian Petrochemical factory in Jiangsu province, China. Moreover, dilute solution of hydrochloric acid (HCl) and sodium hydroxide (NaOH) were employed for pH adjustment and moderation. Analytical grade reagent potassium nitrate (KNO3 ) was used to maintain the ionic strength in zeta potential measurement. Sodium Metasilicate was of analytical grade, purchased from Sinopharm Chemical Reagent Co., Ltd., China. Deionized water (18.25 MΩ) obtained from a Milli-Q Direct 16 (Millipore Q, USA) was used throughout the experiments.
Methods
Micro-flotation tests: The micro-flotation tests were conducted using an XFG-type flotation machine with a 40 ml cell and an impeller speed of 1700 rpm as can be seen in [Figure 2].
Figure 2: Schematic of XFG-type flotation machine.
The Micro-flotation experiments in this study were carried out in a 40 mL-Plexiglas cell. The reagents addition orders and the time of conditioning were as follows: The pure mineral sample (3.0 g) was placed in a Plexiglas cell, which was then filled with 40 mL deionized water (Millipore deionized, resistivity of 18.25MΩ cm). The pulp was conditioned for 3 mins. The depressant was added and then conditioned for 3 mins. The collector was added to the slurry and it was further conditioned for another 3mins before the frother was added to the pulp. After the frother was added to the mixture, it was conditioned for another 3 mins and the flotation was then conducted.
For artificially mixed minerals, the mass ratio of the rutile and almandine (binary mixture) was 1:4 (0.6 g Rutile + 2.4 g Almandine). The impeller speed was fixed at 1700 r/min.The pH of the suspension was modified by the addition of HCl or NaOH as required, after that process, filtration was conducted, and then the concentrate was weighed and dried for final analysis. For single mineral flotation, the mineral recoveries were calculated based on the dry weights of the concentrates (M1 ) and tails (M2 ), see equation (1). The results of each micro-flotation test were measured three times, to sustain the reproducibility, congruity, and repeatability of the experimental parameters and data [34], with the average being reported as the final tally.
Floatability
Where floatability F, is the mineral flotation recovery (%), M1 is the weight of concentrate (g), M2 is the weight of tailings (g) [35]. For artificially mixed minerals flotation, the concentrate was assayed for TiO2 . Rutile recovery (%) was calculated based on the grade (%) of TiO2 and the mass of the concentrate. The equation (2) was used to calculate the above percentage recovery.
Where, Re: the recovery of valuable mineral recovered from raw ores to concentrate, and c is the content of target material in the concentrate (product fraction); t: the content of target material in the tailing (reject fraction); C: mass of concentrate (product fraction); T: mass of the tailing (reject fraction) [36,37].
Contact angle measurements: Solid-liquid contact angle is basically used as a quantitative measure of wettability of a solid by a liquid [38, 39]. The contact angle measurement in this study work was conducted using a Kruss drop shape analysis system (DSA 10-MK2, Germany). The sessile drop method was particularly used to determine the contact angle of water on the pressed mineral pellet surface with a 10 mm diameter. The samples were prepared using the same procedure as in single mineral flotation. The pellets were prepared using 0.3 g of the powdered mineral under a pressure of 2876 psi (196 atm) (ICL International Crystal Laboratories, USA) for 5 minutes. A drop of distilled water would be placed onto the surface of the pressed pellet and a set of microscopic images of the drop and the pellet were taken immediately. Then one image with the momentary view was taken to determine the contact angle by fitting a tangent to the shape of the sessile drop on the microscopic image. The measurements were done in triplicate with average value being recorded as the final.
Zeta potential measurements: The effect of depressant adsorption on surface charges of rutile and almandine particles was examined using a Zetasizernano Zs90 (Nano series, Malvern Instruments, UK). These measurements were based on electrophoresis technique with the equipment being supplied by Malvern Zetasizer Nano (2000). For this, a suspension of the particles was prepared by mixing 0.01 g (? 5 μm size) of the desired mineral with 45 mL of the KNO3 (1 × 10-3 mol/L) as the background electrolyte solution. The pH of the suspension was then modified by use of HCL/NaOH solution for 5 min conditioning time. After the pH adjustment, reagent was added and conditioned for 10 min. After completion of conditioning times, the coarse particles were then allowed to settle for about 5 min. Thereafter, the pH of suspension was noted and the solution containing ultra-fine particles was transferred to a for zeta potential capillary cell for measurements. At least three measurements were made for each experimental condition and the average calculated as the final value at that given condition.
XPS analysis: X-ray Photoelectron Spectroscopy (XPS) is a versatile surface analysis technique used for compositional and chemical states analysis, it can also be employed to provide information and other characteristics of the flotation reagents [40,41]. XPS study has a capability of detecting the chemical composition of the surface and depth of (< 10 nm). In this study work, the XPS data was obtained using a Thermo Fisher ESCALAB 250 XPS system (American Thermo Fisher Scientific).
The samples for XPS tests for both minerals were prepared as following this procedure: 3.0 g single mineral (particle size of 5 μm) was added into sodium metasilicate solution with an appropriate and desired concentration. Then, the pulp was agitated in a mechanical flotation cell for 20 minutes to allow adequate time for effective adsorption process. The stirring speed was basically set at 1700 rpm. Then, the solid samples were washed three times using DI water at the required pH. These washed samples were then vacuum dried at 60°C for more than 12 hours prior to XPS analysis.
RESULTS AND DISCUSSION
Single Mineral Flotation
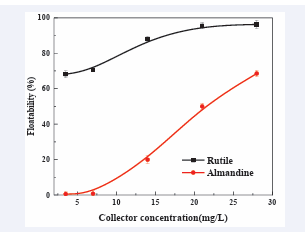
Collector concentration: [Figure 3] shows
Figure 3: Effect of AC1815 concentration on floatability of rutile and almandine. (Pulp pH: 6.0).
the relationship between the collector concentration and the floatabilities for single minerals, rutile, and almandine. As AC1815 concentration increased from 3.5 mg/L to 28 mg/L rutile floatabilities also increased from 68.16% to 95.48% respectively and thereafter a flatten out. The floatability for almandine on the other hand increased from 0.55% to 49.99% at optimum collector dosage. This results were in agreement with the findings [23]. The increase of these floatabilities could have been attributed to promotion floatabilities of rutile and almandine.
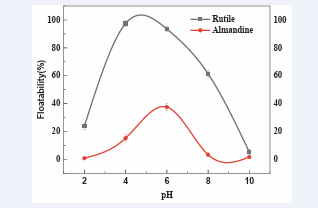
Effect of pulp pH: The action of sodium metasilicate in selective flotation depends mainly on the pH of the pulp/slurry [42, 43]. The results for determination of the best pH for collector is displayed in [Figure 4].
Figure 4: Effect of pulp pH on floatability of rutile and almandine. (AC1815 concentration: 21 mg/L)
In this [Figure 4], AC1815 depicts a good collector performance for rutile floatability at a relatively wide pH range of 2-8. At pH 2 the floatability was at 23.9%, but when the pH was adjusted to 4, the floatability of rutile increased to 97.5%. The increase of pH = 6, the floatability started to decrease and later at pH 10, the floatability recorded its lowest percentage.
Almandine similarly registered the same trend. Floatabilities were very reasonable within the pH ranges 4-6. Consequently, very low floatabilities were registered in too much acidic or too much alkaline environments and conditions of pH 2 (floatability of 0.8%) and pH 10 (floatability of 1.58%) respectively. The optimum results were registered at pH = 4 which recorded the floatability of 37.5% corresponding to good floatability for rutile as well.
We could attribute this outcome to the fact that at those extreme pH conditions, the adsorption of collector (AC1815) onto almandine might have been sharply reduced. Further to the above explanation, the reduction on the collector adsorption on almandine could have been connected to the fact that, in too much acidic condition, mineral surface is positively charged and the cationic collector was also positively charged hence electrostatic repulsion. Also at pH 10, the environment was too much alkaline, cationic collector exists in molecular form, no electrical attraction occurred between collector and minerals surface with highly negatively charged surface [44-46]. Similarly, at pH values above 6, the predominant silicic specimens in solution are basically [SiO (OH)3-] and [SiO2 (OH)2 2-], which could have been specifically adsorbed onto the negative almandine surface forming an insoluble layer which prevented and blocked the collector’s adsorption, and hence leading to decreased floatability.
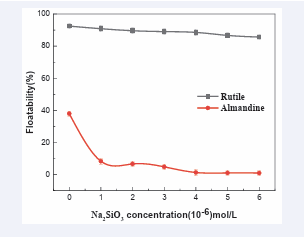
Effect of sodium metasilicate concentration: [Figure 5] shows the
Figure 5: Effects of sodium metasilicate concentration on floatability of rutile and almandine. (AC1815:21 mg/L, pulp, pH = 6).
results of sodium metasilicate influence on single minerals floatabilities. It can be clearly observed that at 0×10-6 mol/L, the floatability of rutile is 92.48% and increasing the depressant dosage to 3×10-6 mol/L, the floatability would still remain above 89%, this clearly indicated that Na2 SiO3 did not have an adverse depressing effect on rutile floatability. Increasing sodium metasilicate further did not register an appreciable decrease in floatabilities of rutile. The floatabilities remained way above 85% at the highest concentration of the depressant (6×10-6 mol/L).
In the absence of sodium metasilicate, almandine floatability was 37.99%. Increasing Na2 SiO3 to 3×10-6 mol/L, the floatability dropped sharply from 37.99% to 4.86% and any further increase of sodium metasilicate beyond the optimum concentration (3×10-6 mol/L), the floatability of almandine was negligible, this clearly shows that the material that was amenable to suppression in flotation system, had already been suppressed. So, it can be decoupled from the results, that rutile and almandine can successfully be separated by the use of AC1815 as a collector and Sodium Metasilicate (SM), as a regulator, as sodium metasilicate has a significant inhibition of almandine floatability, while its inhibition on rutile surface is very low.
Flotation of the Artificially Mixed Minerals
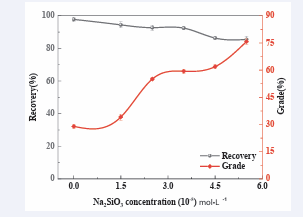
The influence of Sodium Metasilicate concentration on the grade and TiO2 recovery of froth concentrate is shown in [Figure 6].
Figure 6: Recovery and grade of artificially mixed minerals as function of sodium metasilicate dosage. (with fixed total collector (AC1815) dosage of 40 mg/L, pH = 6)).
It is clearly shown that, with the increase of depressant concentration from 0 ×10-5 mol/L, to the 2.5×10-5 mol/L, the recovery initially decreased from 97.79% to 92.72%, and then any further increase of the depressant dosage beyond this optimum dosage didn’t have any noticeable change on the recovery indicating that 2.5×10-5 mol/L was considered as the optimum depressant concentration.
The grade of this flotation process on the other hand increased as the depressant concentration increased, that is, from 28.97% to 55.10% respectively. Dosage concentration of 2.5 ×10-5 mol/L, was therefore considered to be the optimum dosage of sodium metasilicate in mixed minerals flotation, with optimum recovery at 92.72% and the corresponding grade of 55.10%. Further to that, even at high sodium metasilicate concentration, 5.5×10-5 mol/L the recovery remained at reasonably high percentage, that is, 85.50% which was very promising as well. Similarly, the corresponding grade was even very positive at 75.80%. This clearly demonstrated that this depressant was very effective in the flotation separation of rutile from almandine.
Results for Contact Angle Measurements
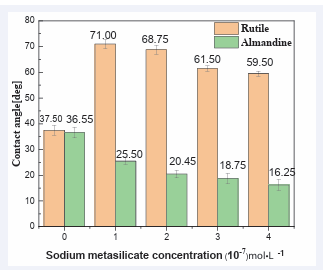
In order to reveal the difference in hydrophobicity between the almandine and rutile, contact angles measurements were conducted. (Figure 7) shows the
Figure 7: Contact angles on mineral surfaces as a function of sodium metasilicate concentration under different conditions: (a) Collector (ACI815) dosage =21 mg/L and (b) pH = 6.0.
measurements of the surface contact angle of rutile and almandine at pH = 6. The contact angles measurements of rutile without flotation chemicals treatment was measured and recorded at 37.5°, a value that concurred with the findings reported in other studies [47-49]. while that of almandine was 36.55°. This indicates that the two minerals are hydrophilic in nature. These results tend to agree with single mineral flotation which exhibited a similar floatability trend for the two minerals when collector was used.
The contact angles of rutile increased to above 70.00° from 37.50°, when treated with depressant and the AC1815 at the concentration of 21 mg/L which changed it from hydrophilic to hydrophobic form. Variations of depressant concentrations did not yield much reduction in the contact angles of rutile. The contact angle of almandine sample after being treated withcollector at the same concentration as that of rutile decreased sharply at various depressant concentrations, proving that the surface of almandine was becoming more hydrophilic than that of rutile when the minerals reacted with sodium metasilicate. The above results therefore suggest that, the hydrophobic difference between the rutile and almandine surfaces was clearly enhanced when both surfaces interacted with the depressant, and consequently their further separation became much easier.
Effects of Sodium Metasilicate on Zeta Potentials of Rutile and Almandine
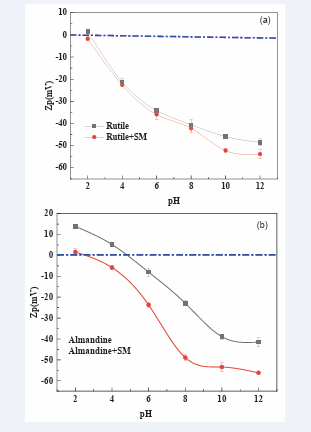
Zeta potential (ζ) is a measure for quantifying the magnitude of the electrical charge at the double layer, additionally it can be used to measure the charges carried by particles that are suspended in an electrolyte solution [50]. Zeta potentials results for rutile and almandine as a function of pH values were determined and are clearly illustrated in (Figure 8a,b) respectively. It can be seen from (Figure 8a) that, the Isoelectric Point (IEP) of rutile before reacting with sodium metasilicate was found to be at pH 2.5. The outcome of these finding were in concurrence with the findings [11-49]. After being treated with sodium metasilicate, the IEP did not occur within the studied pH range. The result for rutile interaction with sodium metasilicate does not display any appreciable change and it can therefore be concluded that the depressant had little effect on the surface of rutile. These results do collaborate closely with the micro flotation test.
On the other hand, the Isoelectric Point (IEP) of almandine mineral as clearly shown in (Figure 8b) was established to be 5.5.
Figure 8: Relationship between Zeta potential and pH value for a. Rutile; b. Almandine.
These results closely agreed with the findings [51]. After treatment with sodium metasilicate, the IEP of almandine became more negative and the IEP pH changed to 3.0. The addition of sodium metasilicate could decrease the Zeta potential values for almandine over the entire pH range tested. The probable explanation to this is the fact that sodium metasilicate negative ions, [SiO (OH)3-] and [SiO2 (OH)2 2-] could have been absorbed on the surface of almandine and thus lowering its zeta potential. This argument closely agree with the fact that the depressant effect of the water glass and its products on the mineral gangue is basically derived from the adsorption of the silicate containing hydrophilic colloids (SiOH)4 on the surfaces of the gangue minerals and hence rendering them hydrophilic [52,53].
It could further be inferred that the depressing effect of SM on the surface of almandine was attributed to two main action mechanism: The first explanation could be due to formation of a stable hydrophilic complex of sodium metasilicate with metal ions from the surface of almandine such Fe3+, Fe2+, since, the active silicates ions in the depressant have a very good capacity of complexation with metal ions, such as iron ions, calcium ions, magnesium ions, and other active metal ions, which generate soluble complexes [23,54-56].
The specimens [SiO (OH)3-] and [SiO2 (OH)2 2-] present in metasilicate solution might have reacted with Fe3+and Fe2+ ions to form the hydrophilic complexes ≡FeH3 SiO4 and ≡FeH2 SiO4-, respectively on the almandine surface which further impaired the collector adsorption. Secondly, sodium metasilicate ions could also get adsorbed on the surface of almandine mineral, and thus changing the electrical properties of its surface. It may be assumed that Fe2+, which is the main active sites in almandine surface, may dissolve significantly in (Na2 SiO3 ) solution. As the negative charges increases with the decrease in Fe2+ on the almandine surface, the electrical property of almandine will be totally altered altogether .The further assumption is that the complexes reactions will proceed as shown in the equations 3-6.
(l) (3)
(l) (4)
To further explain the possible surface complexes which might have led to the depression of almandine floatability, see these ligand-exchange and complexes: ≡Fe-OSi (OH)2 OX or (≡FeO)2-Si(OH)OX for the reactions between silicate monomers and almandine surface. The following ligand-exchange reactions may be proposed as well to expand on the knowledge of the reactions.
(5)
(6)
XPS Analysis of Minerals Treated With and Without Sodium Metasilicate
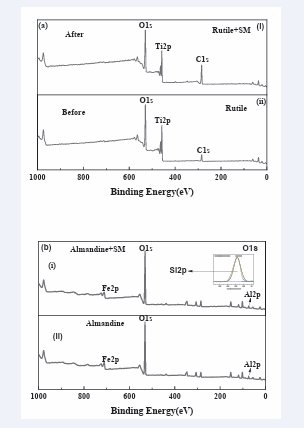
To validate the adsorption mechanism of sodium metasilicate on minerals, the XPS analysis test was conducted. The XPS results of the almandine and rutile treated with and without sodium metasilicate are given in [Figure 9-12] as well as [Table 2,3] respectively. The survey spectrum in (Figure 9a,b)
Figure 9: XPS survey spectra of (a) rutile and (b) almandine in the presence and absence of SM.
clearly revealed that the rutile structure is mainly composed of Ti and O. The C peak (284.60 eV) in the rutile could be due to the surface contaminants formed by exposure to air of the rutile sample and the hydrocarbons from the XPS instrument itself [57,58].
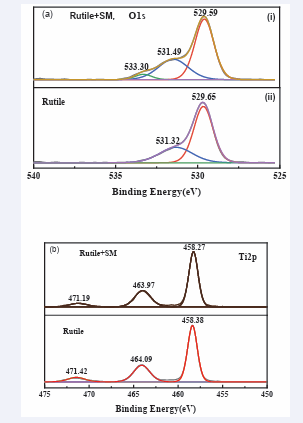
The high-resolution XPS spectra of O, Ti, from (Figures 10a,b), are major part of the oxygen presented on the rutile and adsorbed water surface at all potentials included O2- and OH− species. The XPS spectrum of Ti2 p peaks at potentials of 458.27 eV and 463.97 eV and 471.19 eV treated with SM while peaks 458.38 eV, 464.09 eV and 471.42 eV untreated with SM is shown in (Figure 10b).
Figure 10: High resolutions spectra of (a) O1s and (b) Ti 2p on the rutile surface before and after SM treatment.
Characteristic of these peaks is obviously attributed to the XPS spectrum of Ti2 p in TiO2 [59]. While the peak at 529.59 eV treated with SM and 529.65 eV untreated with the same SM, in (Figure 10a), is basically ascribed to the lattice oxygen in crystalline TiO2 while the other peak at 531.49 eV treated with SM, and 531.32 eV treated with SM in the same (Figure 10a). Could be assigned to the surface hydroxyl groups, physically water and adsorbed oxygen species [60].
The binding energy peaks of iron (Fe), aluminium (Al), oxygen (O) could be detected on almandine surface respectively. Some of traces of O and C present in the almandine surface could be attributed to external pollution of instrument and atmosphere as well [58]. (Figure 11a,b),
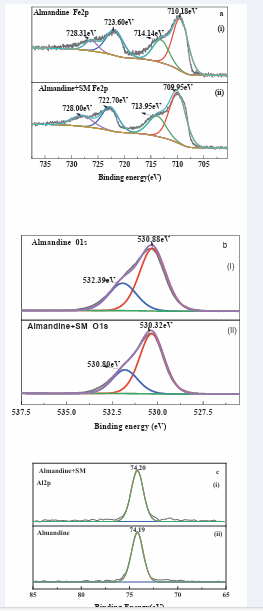
Figure 11: High-resolution XPS spectra of a. Fe 2p, b. O 1s and c. Al 2p on almandine surface before and after treatment with SM.
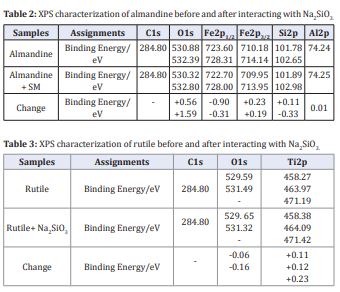
and c present the Fe2 p, Al2 p and O1s spectra of the almandine before and after treatment with SM respectively. Consequently, the Al(2p) spectrum is shown in (Figure 11c (i)). Exhibits a peak at 74.20 eV when treated with reagent SM, which is basically ascribed to Al2 p3/2 in Al-OH/O at the surface of almandine [61,62].
The binding energy before SM treatment was 74.19 eV in (Figure 11c (ii)), this reaffirms very negligible change of 0.01 eV clearly indicating that the element Al in the almandine surface does not react with any species of (Na2 SiO3 ). In (Figure 11a), Fe2 p of untreated surface show peak of 710.18 eV which is assigned to Fe2 p3/2 suggesting coexistence of 2 Fe+ and 3 Fe+which are the active elements in almandine mineral [63,64]. In the same (Figure 11a), the peaks located at 710.18 eV and 723.60 eV corresponded to the binding energy of Fe2 p3/2 and Fe2 p1/2 [65, 66].
However, after being treated with the depressant at pH = 6, this broad band shifted to 709.95 eV binding energy, which may be assigned to elements, 3 ( ) FeOH , 2 3 FeO or 2 4 FeOand FeOOHamong others which are collectively referred to as iron ‘ox hydroxide’ [67,68]. These elements form the bulk of hydrophilic layers which are basically associated with the depression of almandine. Peak 713.95 eV may also be ascribed to FeOOH[69], which may mean that after reaction sodium metasilicate, almandine formed a hydrophilic layer making it impossible for the adsorption of the collector. The other elements in the reaction with depressant are: 714.14 eV which is assigned to Fe2 p3/2 as well. The change in the peaks exhibits a chemical change. The peaks which were observed at; 723.60 eV and 728.31 eV are basically attributed to Fe2 p1/2 . Based on the variation in the binding energy of peaks it can conclude that there was a chemical adsorption of depressant onto the surface of almandine. The changes are clearly depicted in (Table 3,4). The peak of O1s in (Figure 11b) without 2 3 NaSiO treatment was 530.88 eV which might be ascribed to iron ox hydroxide belonging to 3 ( ) FeOH and 3 2 4 3 ( ) FeAl SiO [70,71]. After deconvolution and the treatment with the depressor the new peaks appeared at 530.32 eV, which is assigned to [72,73]. The peak at 532.80 eV before treated with depressant changed to 530.39 eV after the treatment and this is basically ascribed to Fe2 SiO4.
(Figure 12a,b) displays the
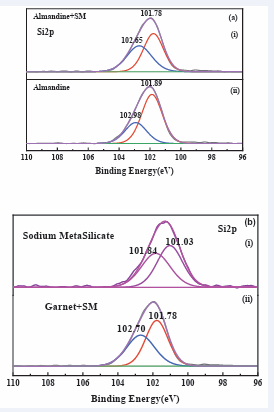
Figure 12: High-resolution XPS spectra of (a) Si2p, on (b) almandine surface before and after treatment with sodium metasilicate.
Xps test for element Si2 p in the sodium silicate depressant and how it interacted with almandine mineral gangue. The binding energy of the Si2 p peaks before depressant interacted with almandine is located at 101.03 eV and 101.84 eV, after the interaction with sodium metasilicate the peaks shifts to 101.78 eV and 102.70 eV which indicated that there was the formation of pure iron silicates [40], which are hydrophilic in nature and produced the depression of almandine surface. It can be therefore inferred that a (Si2 p) element in SM, interacted with almandine surface through chemical bonding to cause depression of the almandine floatability.
CONCLUSION
The results of single micro flotation tests revealed that almandine and rutile had similar floatability characteristics when AC1815 was utilized as the collector that is, the floatabilities increased as the collector concentration was increased in both cases. Similarly, zeta potential results on the other hand, have demonstrated that adsorption of anions of either [SiO(OH)3-], [SiO2 (OH)2 2-] and [SiOH-] might have played a critical and key role in the depression of almandine under flotation pH range (pH = 4-8) through electrostatic force. Depressant-almandine complexes might have taken place in form of ligand-exchange: ≡Fe-OSi(OH)2 OX or (≡FeO)2-Si(OH)OX which could have contributed further to depression of almandine floatability. Contact angle measurements demonstrated that the surface of almandine became more hydrophilic than that of rutile when both minerals were treated with sodium metasilicate.
A further observation from XPS analysis was that, a (Si2 p) element in sodium metasilicate interacted with almandine surface through chemical bonding to cause depression of the almandine floatability. Finally, the adsorption of sodium metasilicate in almandine surface through chemisorption might have caused the depression of its floatability by reducing the number of Fe active sites for collector attachment and adsorption.
ACKNOWLEDGEMENT
This work was funded by the Natural Science Foundation of China (projects Nos.51904208, 51974205) and The Open Foundation of State Key Laboratory of Mineral Processing (BGRIMM-KJSKL-2021-16). This research is also supported by International Science and Technology Cooperation Program of Hubei Province (project No.2022EHB016).