Cathode Materials of LiNixMnyCo1-X-YO2 Form Battery Recycling Reproduction
- 1. Yenchun Liu
Abstract
This study retrieved from spent lithium nickel cobalt batteries, lithium battery cathode material made again. The battery assembly and battery charge and discharge tests. So can waste resources and reduce environmental pollution and reduce the battery cell manufacturing costs. Currently lithium battery recycling waste processing method is the use of hydrometallurgy, the lithium, cobalt, nickel, manganese and other elements separate one by one. Were made from a variety of pure oxides or carbonates, complicated process, economic efficiency as expected. The study will capture waste batteries cobalt, lithium, nickel, made of lithium nickel manganese cobalt cathode ternary material powder (LiNix Mny Co1 -x -y O2 ). Analysis of the proportion of the process solution of lithium, nickel, manganese, cobalt, add the lack of metal ion ingredients. After adjusting the correct composition of cathode electrode material powder sintering. Thus eliminating the need for separate elements of procedures and the cost can be greatly reduced. Reproduction of the cathode material. That SEM and XRD is observation of the fine structure and organization and finally assembled into a battery. The battery charge and discharge performance test can reach 122.21 mAh / g and 85% is about the pure raw materials production. So it can reduce the manufacturing cost of the cathode material. The waste of valuable battery cobalt and other materials to be recycled to reduce waste and resource the head.
Keywords
• Lithium battery
• Cathode material
• LiNix Mny Co1 -x-y O2
• Charge and Discharge
Citation
Liu Y, Liu M (2025) Cathode Materials of LiNixMnyCo1-X-YO2 Form Battery Recycling Reproduction. J Materials Applied Sci 6(1): 1014.
INTRODUCTION
Lithium battery cathode material divides in four types, lithium manganese, lithium-nickel-cobalt, lithium nickel cobalt manganese and lithium iron phosphate [1-5]. Lithium manganese are cheapest raw materials, but these materials have lower electrochemical capacity and life expectancy is shorter. Therefore the lithium manganese department frequently use the powder of lithium nickel cobalt to mix because resources of cobalt on earth are very low. The price of cobalt is quite expensive and there are toxic if used cobalt lithium battery. It will not be recycling so that it will not only wasteful of cobalt would also pollute the environment. Lithium-nickel-manganese-cobalt powder is newly developed ternary material of cathode material. Its properties depending on the relative amounts of the fixed rate fast charge-discharge long life, but the unstable life will be short, the nickel cobalt manganese is ancient content and has much thermal stability and low-cost.
The reasons affecting the capacitance caused by various lithium-ion battery materials have been widely researched. These include the dissolution of Mn in LiMn2O4, which has the greatest impact on the LiMn2O4 reversible capacitance. An electrolyte (LiPF6) will react with water molecules and enable Mn to be dissolved. The Mn ions will support trivalent and tetravalent conversion of the Jahn- Teller effect at the same time, and the solvent molecules and the anode surface will generate a passivation film because of charge attraction. Them over charging will cause the electrolyte reduction and battery self- discharge phenomenon, and these all will cause the decline of the capacitance [6,7]. The improvement method, Wakihara and others have replace the magnetic ion in LiMn2O4 with nickel or chromium ions to increase the electrochemical stability [8,9].
In Lithium battery oftenly used cathod material is lithium manganese (LiMn O ) and lithium- nickel-cobalt (LiNixCo1-xO2) mixed use. The price is cheaper than lithium manganese, but life is short. The lithium-nickel-cobalt is more expensive, life is more long than lithium manganese. The mixed up them together can reduce raw material costs and also has a quite important point is the moisture that in the electrolyte contains can make LiPF6 decompose releases HF. This HF will corrode, this lithium manganese-based cathode material and lithium-Nickel- cobalt. when the lithium nickel cobalt is the use will have few decompositions. The release lithium ion and this lithium ion just can neutralize HF to produce harmless LiF, will not continue to corrode. The lithium manganese is the anode material endangers the life of battery if can develop a cheap process. The lithium battery anode material makes commonly used solid state reaction method [10-14], but abandons the processing method of lithium battery recycler at present is to use hydrometallurgy [16-17]. Research in recent years to improve the efficiency of the battery continues, for example enhancement of cathode capacity utilization through a novel concept of material design. Layered transition-metal oxides have served as the mainstream cathode materials for high-energy batteries due to their large theoretical capacity (∼280 mAh/g) [18]. The processing − structure − property relations in
≤ y ≤ 0.6 were investigated [19]. The Lithium battery to separates lithium, cobalt, nickel, manganese and other elements. To makes various pure oxides or thecarbonates separately process complicated. The cost is not cheap. The plan did not use the element separation law that the common manufacturer uses. The makes the lithium nickel manganese cobalt three composition material anode material powder directly and solution analysis the proportion of lithium nickel manganese cobalt. The add shortage of ingredients made directly positive electrode material powder to eliminating the element separation procedures. Also, The Ni-rich lithium nickel manganese cobalt oxide cathode materials of synthesis methods and their electrochemical performances be reported [20].
Since the lithium battery materials industry price competition between each other very violent. When are cheaper sources of raw materials. It will stand the competition unbeaten status. Cost of lithium battery cathode material cobalt accounted for the largest cost. That lithium and nickel, if we can develop a cheap process. To choose lithium nickel cobalt from waste batteries and it will be a large reduction in manufacturing cost of lithium battery cathode. Characteristics of the study in addition to not using the element separation. Also will make use of combustion cathode electrode material powder. The characteristics of this method is a powder made of a solid-state reaction method than the traditional tiny and more suitable for high-current discharge. We also dope with a transition metal, nickel, to synthesize LiNixMnyCo1-x-yO2 and further to explore nickel effect on the lithium ion battery cathode materials’ electronic and chemical-electronic characteristics. The aim is to obtain the optimum preparation conditions and parameters forhigh capacitance and to improve the cycle performance of battery materials.
EXPERIMENT SECTION
Materials and measuring tools
Lithium carbonate (Li2CO3), Nickel Oxide (NiO), manganese tetraoxide (Mn3O4), 1-methyl-2- pyrrolidone (NMP), polyviylidefluoride (PVDF) and other reagents were all G.R. grade chemicals for synthesis. Experimental equipment were a computer-interface X-ray powder diffractometer (XRD) with Cu Kα radiation (Rigaku D/ Max-II) used to identify the crystalline phase. Particle morphological feature was imaged by scanning electron microscope (SEM, JEOL JEM 200CX) with an accelerating voltage of 15kV. The battery automation test systems of BAT-750 series (Acc. Tech. System Co.,) was used for charge capacity test.
Preparation of zinc and iron oxide magnetic powder
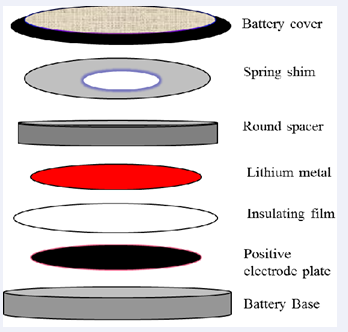
Pre-treatment of waste lithium batteries Lithium battery positive and negative, respectively the copper foil to a substrate. The positive and negative active material is coated. The substrate fluid as primers is leads to the current outside the battery. Each lithium foil outsourcing there are dozens and dozens of positive and negative from overlap between the positive and negative electrodes separated by a spacer material to make insulation of the capacity of 36wh. Stacked together to form a plurality of lithium battery, its shape as shown in Figure 1.
Figure 1: Waste lithium appearance
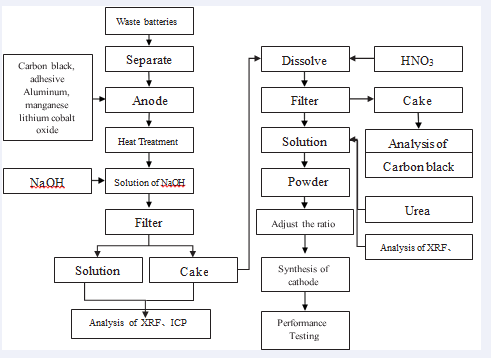
First dismantling waste lithium battery of the positive and negative electrodes separately. Then put into a solution of sodium hydroxide in the positive electrode. The aluminum foil substrate is dissolved in the positive electrode. The positive electrode powder applied to the aluminum foil by being alkali-insoluble filtration. Then separating the positive electrode powder dissolved in nitric acid of into lithium cobalt-nickel-manganese nitrate solution. Toner and PVDF adhesive powder in the cathode electrode due to insoluble in nitric acid is isolated by filtration.
The preparation of LiNixMnyCo1-x-yO2 anode material
Analyzes in the solution with ICP the lithium. The nickel, manganese, cobalt content is calculates must manufacture the lithium. The nickel, manganese, cobalt ionic weight from the solution which 333 materials (LiNi0.3Mn0.3Co0.3O2), 442 materials (LiNi0.4Mn0.4Co0.2O2) and 532 material (LiNi0.5Mn0.3Co0.2O2) needs to make up. To compensate for the calculation of volatile calcination process of lithium, excess lithium to 5%. Then calculate the required according to lithium carbonate, nickel nitrate and manganese nitrate of cobalt nitrate added to the solution. Then add urea in solution when reduction agent then boil. There are nitrate solution (oxidant) and urea and burst into flames to the solution boiled away into ashes when after grinding. This ash is the desired lithium battery cathode material powder. The adhesive (PVDF) and a conductive agent (graphite) and NMP solvent was added to this powder and stirred into a slurry. The slurry is coated on an aluminium foil to made of the positive electrode. The lithium metal as a negative electrode and then to prepare a coin-type battery.
The test the system features positive electrode material and the experimental process shown in Figure 2.
Figure 2:Lithium battery cathode waste processing
Lithium battery assembly and testing
The lithium battery cathode material powder adhesive (PVDF) and conductive agent (graphite) and NMP solvent was added lithium stirred into a paste, coated on aluminium foil, made positive. Then the lithium metal as the anode and made button button-type battery cathode materials testing system characteristics. The experimental process shown in Figure 3.
Figure 3: Button-type battery assembly flowchart
The finished button-type battery with 8 channels charging-discharging testing device was connected a to conducting battery charging and discharging test. The first stage was to charge the battery at a constant current (C/20), after which, the voltage was raised to 4.5V. A constant voltage 4.5V was used for the second stage charging until the current was less than 0.01mA. Either constant current charging or constant voltage charging was used until reached. When the battery was fully charged, the third stage was to discharge at constant current (C/20) until the voltage had dropped to 3V.
RESULTS AND DISCUSSIONF
XRD analysis LiNixMnyCo1-x-yO2 cathode material
That are to manufacture 333 materials, 442 materials and 811 materials. The ICP analysis again by NiO, CoO, Li CO , nitric acid and urea quantity that each unit waste lithium battery anode material needs. When is the counter- balance shrimp burns the evaporation of lithium. The use of lithium carbonate has 5% excesses. The response between nitric acid and urea is as follows:
6HNO3 + 5(NH2)2CO → 8N2 + 13H2O + 5CO2
Solution due to nitrate (oxidizer) and urea.The reaction is a redox reaction and emit a lot of heat, so that the solution on fire to so far has been to ashes. Synthesis sintering temperatures were 825?, 850?, 875?, 900?. Figure 4
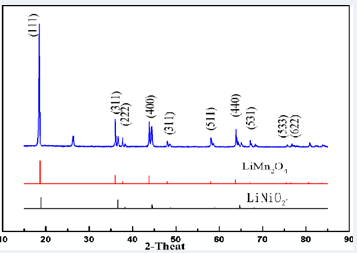
Figure 4:XRD pattern of cathode material for waste lithium battery.
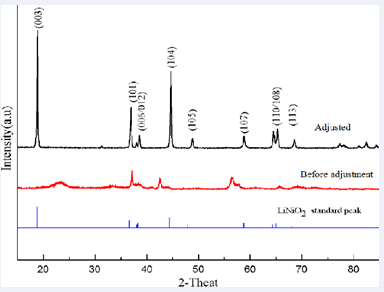
is the X diffraction chart of waste lithium battery anode material. Its primary structure is the spinel structure (LiMn2O4) and layered rocksalt structure (LiNiO2) . Calcinated after burning 333 ternary cathod material of powder x - ray diffraction diagram. This figure has not seen the spinel structure and only pure layered rock salt structure. Figure 5 is calcined after 333 ternary material cathode material powder XRD pattern. That can be seen from out of diffraction angle in 36.6o, 38.1o, 38.3o, 44.4o, 48.9o, 58.8o, 64.5o, 64.7o and 68o of diffraction peaks and the underlying the standard normalization agreement. That the product has a single layered LiNiO2 rock salt structure and no impurity phase. This result comparison with ICP analysis results were consistent with the structure of LiMn2O4 66mol% and LiNi0.6Co0.4O2 34mol%. Waste lithium batteries made LiNi0.33Co0.33Mn0.33SEM morphology is seen from 825 ?. That have irregular shape and grain flaky primary particles have been formed. To improve the LiMn2O4 charging- discharging cycle, appropriate doping of Ni and Co into pure LiMn2O4 is necessary. However, the transition metal does not participate with lithium-ions embedded in and out of the electrochemical reaction. Therefore improving the reversibility of charging and discharging, decreases the capacitance, but improveing cycling characteristic, andhelp lithium- ion to increase chemical diffusion coefficient.
Figure 5: XRD pattern of the cathode electrode material powder produced
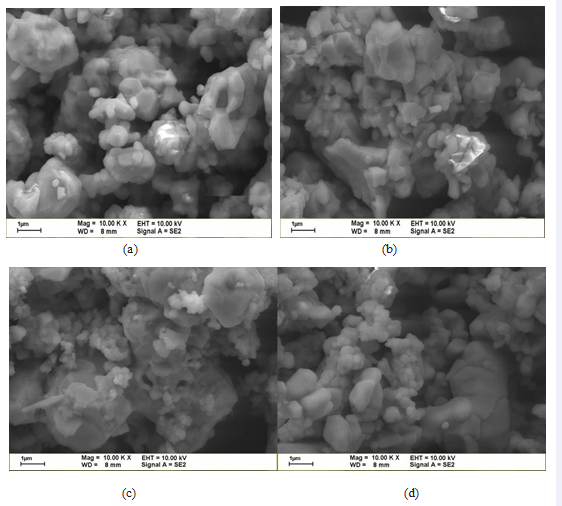
SEM analysis LiNixMnyCo1-x-yO2 cathode material
The particle size distribution is relatively loose and
Figure 5:XRD pattern of the cathode electrode material powder produced
irregular arrangement. Primary particles bound more closely and more smooth particle surface. The particle size increases and generates agglomeration. The SEM morphology as shown in Figure 6 (a) ~ (d) can see sintering temperature in 825? to 900? out of powder. Particle diameter about at about 2 to 3 μM size. When the temperature increased to 900? and not because temperature increases with increasing particle path followed. This can avoid the problems from the solution method in which there are more impurities and they are harder remove. Thus the solid-state reaction synthesis method is still better for battery material quality. The SEM observation shows the surface morphology results: when the temperature is raised to 825? the particles will have aggregated.The results seen in the synthetic powder temperatures of LiNi0.33Co0.33Mn0.33 O2 powder at 825?, 85?, 875? and 900? do charge-discharge test. The result can see discharge capacity reached to 120 mAh /g when sintered at 850? as shown in Figure 7.
Figure 7: Discharge capacities of LiNi0.33Co0.33Mn0.33 O2 cathode material made from waste lithium battery
We have examined the correlation charge- discharge characteristics of the LiNi0.33Co0.33Mn0.33 O2 prepared at different heat- treatment temperatures from 825 to 900?. It was found that the LiNi0.33Co0.33Mn0.33 O2 heat-treated at 850°C possesses a tremendously high charge-discharge capacity of formula. The method of waste battery treatment and price of raw materials of battery nickel, cobalt, lithium and magnet is low. That is high additional value of product to avoid a lot of material consumption the nickel, cobalt and manganese separation required. While manganese metal recycling metal recovery rate of 98.5% to produce nickel cobalt lithium magnate the cost can be reduced about 20% than using new raw materials. The reducing the damage of waste batteries to the environment to achieve battery material product form reduction. The waste and green resources related to technologies. This suggests that there is cathode mechanism for the charge-discharge reaction besides a LiNi0.33Co0.33Mn0.33 O2 compound mechanism which is well known. The initial discharge capacities for
the cells cycled at elevated temperatures was found to be higher when compared with the cell cycled at 850°C. Synthesized LiNixMnyCo1-x-yO2 were then heat treated at 850 °C. X-Ray Diffraction patterns showed well defined splitting of [006]/[102] and [108]/[110] diffraction peaks indicating layered structure and good hexagonal ordering [21].
Various different proportion and initial discharging of used batteries
Waste batteries made LiNi1/3Co1/3Mn1/3O2 cathode material. That LiNi0.33Co0.33Mn0.33O2 (333 materials), LiNi0.4Co0.4Mn0.2O2 (442 materials) and LiNi0.8Co0.1Mn0.1O2 (811 materials) is to Measure discharge capacitance seen from Figure 8 at 850? excess lithium same time 4%. The LiNi0.8Co0.1Mn0.1O2 is with higher capacitance 138.83 mAh / g. The result is shown in Figure 8. Purematerial preparation 333 material, 442 and 811 material the initial discharge capacity between 133.64 ~138.83 mAh /g. The use of waste battery processed materials for lithium ion battery capacity up to 122.21 mAh / g up to 88%. However, the LiNi0.8 Mn0.1Co0.1O2 ,separation of elements and then by mixing for calcination procedures. The method is simple lot, capacity and commercial chemical powder resulting powder is extremely similar. The XRD before the hetero phase is not added and calculated by adding after shows the product has a single LiNiO2 layered rock salt structure. The produce no impurity phase. Reproduction of waste batteries cathode material experiments capture cobalt, lithium and nickel from waste batteries. That will reduce the cost of manufacturing large convergence lithium battery cathode materials. The analysis of lithium, nickel, manganese, cobalt proportion to the solution and adding insufficient metal ion ingredients. This made directly to positive electrode material powder and sintered at 850? discharge capacity may reach 122.21 mAh / g of about 85% of pure raw materials production. The manufacturing cost of the cathode material greatly reduced. Which can also contain the value of the cobalt and other materials to be recycled, to reduce waste and resource waste.
ACKNOWLEDGEMENT
The financial support from the National Science Council under grant number MOST 104-2221-E- 274-003 and MOST 105-2221-E-274-003 is gratefully acknowledged.
REFERENCES
- Ewais EM, Mahmoud MM. Abdel-Hady EA. In-Situ synthesis of Magnetic Mn-Zn Ferrite ceramic object by solid state reaction. J Aust Ceram Soc. 2008; 44: 57-62.
- Zheng ZG, Zhong XC, Zhang YH, Yu HY. Synthesis structure and magnetic properties of nanocrystalline ZnxMn1-xFe2O4 prepared by ball milling. J Alloys Compd. 2008; 466: 377-382.
- Ammad QH. The influence of hafnia and impurities (CaO/SiO2) on the microstructure and magnetic properties of Mn-Zn ferrites. J Cryst Growth. 2006; 286: 365-370.
- Upadhyay C, Verma HC, Rath C, Sahu KK, Anand S, Das RP, et al. Mossbauer studies of nanosize Mn1-xZnxFe2O4. J Alloys Compd. 2001; 326: 94-97
- Arulmurugan R, Vaidyanathan G, Sendhilnathan S, Jeyadevan B. Mn- Zn ferrite nanoparticles for ferrofluid preparation: Study on thermal- magnetic properties. J Magn Magn Mater. 2006; 298: 83- 94
- Vogler C, Butz A, Dittricj H, Arnold G, Mehrens MW. Electrochemical and structural comparison of doped lithium manganese spinels. J. Power Sources. 1999; 84: 243-247
- Liu YC, Liu MC, Robert Lian Huey Liu, Chi MC. Effect of the Doping Ni and Overdosing Lithium for Synthesis LiMn2O4 Cathode Material by the Solid State Reaction Method. Appl Mech and Mater. 2014; 457- 458, 93-97.
- Wakihara M, Guohua L, Ikuta H, Uchida T. Chemical diffusion coefficients of lithium in LiMyMn2 − yO4 (M = Co and Cr). Solid State Ion. 1996; 907: 86-88.
- Liu YC, Liu MC, Chi MC. Optimal Charge and Discharge Capacity Effects of the Sintering Process on LiMn2O4 by the Solid-state Reaction Method. Appl Mech Mater. 2013; 377: 141-144.
- Rath C, Sahu KK, Anand S, Date SKN, Mishra CR, Das P. Preparation and characterization of nanosize Mn-Zn ferrite. J Magn Magn Mater. 1999; 202:77-84.
- Mane DR, Patil S, Birajdar DD, Kadam AB, Shirsath SE, Kadam RH. Sol-gel synthesis of Cr3+ substituted Li0.5Fe2.5O4:cation distribution, structural and magnetic properties. J Mater Chem Phys. 2011; 126: 755-760.
- Song F, Liu X, Shen M, Xiang J. Preparation and magnetic properties of SrFe12O19/Ni0.5Zn0.5Fe2O4 nanocomposite ferrite microfibers via sol-gel process. J Mater Chem Phys. 2011; 126: 791-796.
- Pozo GL, Silvetti SP, Aguirre MC, Condo AM. Synthesis and characterization of (NiZnFe2O4)0.5/(SiO2)0.5 granular nanocomposites. J Alloys Compd. 2009; 487: 646-652.
- Lu X, Zhou T, Jia M. Hydrothermal synthesis of Mn-Zn ferrites from spent alkaline Zn-Mn batteries. Particuology 2009; 7: 491-495.
- Saezpuche R, Torralvofernandez MJ, Gutierrez VB, Gomez R, Marquina V, Marquina ML, et al. Ferrites nanoparticles MFe2O4(M=Ni and Zn): hydrothermal synthesis and magnetic properties. Bol Soc Esp CeramV. 2008; 47: 133-137.
- Y´anez-Vilar S, S´anchez-And´ujar M, G´omez A, Mira JM, Castro- Garcia SA. Simple solvothermal synthes of MFe2O4 (M=Mn,Co and Ni) nanoparticles. J Solid State Chem. 2009;182: 2685-2690.
- Liu YC, Chi MC. Lin CM. Reaction model correlation of the 2,3,5-trimethyl-1,4-benzoquinone synthesis using CuFe2O4 nano- powder as the catalyst. Int J Chem Eng Appl. 2015; 6: 42-48.
- Wu Z, Xiaogang H , Jiaxin Z , Yi W , Ruimin Q , Fei S , et al. Depolarized and Fully Active Cathode Based on Li(Ni0.5Co0.2Mn0.3)O2 Embedded in Carbon Nanotube Network for Advanced Batteries. Nano Lett. 2014; 14: 4700- 4706.
- Xiao J, Natasha A. Chernova, M. Stanley Whittingham, Influence of Manganese Content on the Performance of LiNi0.9−yMnyCo0.1O2 (0.45 ≤ y ≤ 0.60) as a Cathode Material for Li-Ion Batteries. Chem Mater. 2009; 22: 1180-1185.
- Farish IS, Muhd FK, Tan W Kelimah AE, Azira A, Nurul DB, Muhamad KY, et al. Ni-rich lithium nickel manganese cobalt oxide cathode materials: A review on the synthesis methods and their electrochemical performances. Heliyon. 2024; 10: 1-24.
- Berke P, Kadri A. Development and characterization of layered Li(NixMnyCo1−x−y)O2 cathode materials for lithium ion batteries. Int J Hydrogen Energ. 2016; 41: 9852-9859.