Fabrication of Aluminum Nitride Powder from Scrap Aluminum Foil
- 1. Graduate School of OptoMechatronics and Materials, WuFeng University, Taiwan
Introduction
Application of aseptic package is extensive, it may be divided into two types, one is coated with an aluminum foil on both surfaces of the PE film; other is not coated with a PE film, but also mixed with paper. Aluminum foil coated with PE film is light-weight, used in packaged of food, machinery electronic parts, pharmaceuticals and so on. It contents about 40% of aluminum foil and 60% of PE resin. The PE film provides heat sealing and aluminum foil provides no ventilation. The other type of the aseptic pack is aluminum foils mixed with paper most used to hold fruit juice and milk. Based on the needed of the strength, there may be multi-paper layers, one layer of paper sheet, one layer of PE resin stacking together to form the outer layer, the middle layer is aluminum foil, the innermost is PE film, the aluminum contents about 20% and PE contents about 25 %, and 55% of paper. Thick aseptic packaging provides mechanical strength and printability; PE layer provides sealing; and aluminum foil provides no ventilation. Use aseptic packaging so that the food will not be eroded bacteria and lead to corruption. Food containers are made of paper, foil or paper coated aluminum foil inside it. These containers have a common characteristic, no matter a foil or paper, all coated with a polyethylene film on its surface to provide heat-sealing properties and water-resistant container. Aluminum foil or paper with a PE film may be formed as an impermeable, no ventilation food containers after a hot pressing process. Containers made by paperare lunch boxes, cold/hot water cups, instant noodles bowl and milk container and so on. Containers made by Aluminum foil are fast food packages, medicine packing aluminum foils for drug sealing to avoid from the moisture. Paper containers containing aluminum foil which used in juice packages, etc.
“How to turn the huge amount of abandon aluminum foil into recyclable sources to produce valuable electric materials” has become a meaningful study in the fields of energy, environment, and sustainable development. This study aims to the reproducing aluminum nitride which combines its atoms by covalent bond and its structures is hexagonal crystal wurtzite, providing the high melting point and great thermal conductivity. Due to the high coefficient of thermal conductivity and electrical insulation properties, and is a solid metal-free, aluminum nitride (AlN) is usually applied to the electronic board, such as a thermal paste or a heat sink pad robber propagating through a high thermal conductivity of aluminum nitride (AlN) powder is made into a liquid or solid resin, respectively. Suitable to LED, CPU and high-power transistors heating element and a heat sink, a heat pipe or liner as to increase the contact area of the heat sink is made of sintered powders of high thermal conductivity is formed and having better heat removal. The high density of high-power transistor, LED, and the CPU will generate more heat, and result in higher demand of radiator products, such as the thermal grease and the thermal pad robbers. And the development
of photovoltaic industry in recent years. The future of the AlN nanoparticles is enthroned the mainstream products for the heat sink, packing, piezoelectric, and field emission materials so on [1]. Currently, there are two methods for AlN synthesis [2-6], one is produced by direct nitridation, and the other is produced by reducing nitridation of aluminum. Direct nitridation is using aluminum material which has large surface area, e.g. aluminum powder, to process the nitridation at the high temperature nitrogen ambient. Reducing nitridation is to process the nitridation of aluminum oxide and carbon powder alloys under the high temperature nitrogen ambient. Although lower nitriding method of reducing costs, but its quality is even worse, because of the carbon remains
Al + N2 → AlN (1)
Al2O3 + N2 + 3C → 2AlN + 3CO (2)
Kameshina used mechano-chemical treatment on low temperature synthesis of AlN from Al metal in N2 atmosphere [7]. Lee used the combustion synthesis(SHS) method for the production of AlN powder [8]. Hong used wet ball milling of aluminum foil crap to fabrication of aluminum flake powder [9]. Other relevant research techniques are: Lin used aluminum powder to synthesize AlN with N2 0.4 MPa and obtained a high producing rate [10] in 2001. In the producing process, Lin added NH X, CO(NH ) , CH (CH ) COOH, and CO H(CH ) COOH.Gromov produced Al3O3N and AlN, and recorded the burning processes clearly by burning the Al-Al2O3 and 0.5~9 µm aluminum powder without other additives [11-13]. The aluminum nitride substrate normally can be getten by liquid phase sintering to increase mechanical density or remove dissolved oxygen in AlN crystal particles which is not good for thermal conductivity. In a large number of sintering additives, the rare earth oxide raw materials, an alkaline earth metal oxide material, carbide powder and carbon have increased density by liquid phase sintering of mechanical [14,15]. Yttrium oxide and calcium oxide are effective sintering additive, and may have a value of aluminum nitride plate and the thermal conductivity is higher than 150 W/mK. Liu Min- Chi used combustion method, and achieved concrete results from recycled aluminum foil [16].
EXPERIMENT
Starting materials and measuring tools
Xylene, hydrogen chloride (HCl), sodium hydroxide (NaOH), sulfuric acid (H2SO4) and the other reagents are chemically synthesized levels of GR. The experimental device is a solvent extraction tank equipped with a stirrer and temperature control functions, dissolved, and then precipitated PE plastic powder, canister filter with plastic powder, paper and aluminum foil.
PE powder particle size was observed with an electron microscope and the use of FTIR to see if there is residual xylene in the presence of plastic. The computer-interface of powder X-ray diffraction (XRD) with CuKα rays (Rigaku D / Max -II) for identification to the crystal phase. Imaging particle morphology by scanning electron micrograph (SEM, JEOL JEM200CX) with an acceleration voltage of 15kV.
Scrap aluminum foil recycling procedures
In this study, the solvent extraction method, cannot be dissolved in the solvent at room temperature with xylene application properties of PE, but soluble when the temperature exceeds 60?.
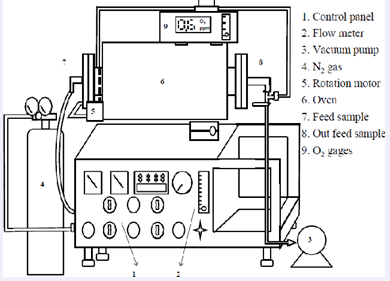
Using xylene as a solvent and an aluminum foil or paper waste into xylene and heated, aluminum foil or waste paper of the polyethylene film is dissolved, and then the aluminum foil and PE or paper will be separated. Nitriding the aluminum foil into the vacuum furnace, heated to 1270?, and nitrogenizing4 hours in a nitrogen atmosphere, as shown in Figure 1.
Figure 1: Equipment of atmosphere sintering oven
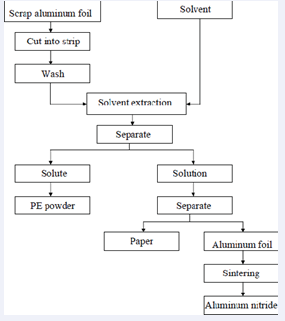
Then crushed and ground to obtain an aluminum nitride powder. An aluminum nitride powder is added 1% of Y2O3 was grinded, molded and finally sintered in a nitrogen atmosphere, carried out at 1500? 2 hours to obtain an aluminum nitride plate. The aluminum nitride powder and plated will be inspected by X -ray diffraction analyzer(XRD), scanning electron microscopy(SEM), thermal conductivity coefficient and surface area measurement instrument for their characteristics. The processes for using waste aluminum foil to produce aluminum nitride were shown in Figure 2 below.
Figure 2: Scrap aluminum foil recycling procedures
RESULTS AND DISCUSSION
Waste aluminum foil recycling technology
Using xylene as a solvent, a solvent such as xylene-based PE at different temperatures of different solubility [17]. The scrap aluminum foil first layer of the container is paper, the second layer is PE film, the third layer is aluminum foil, the most inner layer is PE film. Currently, the test results are very successful and high economic value, however, the use of xylene as a solvent, does have problems. Its fluid pint too low (only 20?) can easily cause a fire, if you use a higher liquid pint of solvent, it would be more secure. Beside, the solubility of PE resins in xylene at 100? only around 8 %, if a higher solubility of the solvent is used, the need of solvent amount will be smaller. That is, the output will be much more when using the same equipment. The built of solvent extraction equipment were shown in Figure 3
Figure 3: Equipment of solvent extraction
below. The principle of scrap aluminum foil dissolved into PE resin is semi-crystalline material at room temperature. The powerful intermolecular force made there is no solvent molecule can enter the crystal to dissolve PE resin however, when the temperature increased to 80?. The crystal is destroyed, many hydrocarbon solvents can dissolve PE resin; after the temperature is decreased, the PE resin inside the solvent are re-crystallizated. Using filter to separate of plastic powder, paper and aluminum foil, shown in Figure 4 below.
Figure 4: Scrap aluminum foil recycling of (a) Aluminum foil (b) Paper
(c) Plastic powder
Based on the nature of aluminum foil with a huge surface area, made from aluminum foil to aluminum nitride may increase the added value several times. The method of nitriding an aluminum foil, the foil is placed in a vacuum furnace, heated to 1270?, under a nitrogen atmosphere for 4 hours. Re-grinding and milling, to obtain an aluminum nitride powder, and then by grinding, forming, and finally raised to 1500? were sintered nitrogen for 2 hours. Then the aluminum nitride plate was obtained. In the study, the results are the same either made by aluminum foil or aluminum powder, the material cost is much lower.
Preparation and properties of aluminum nitride
“How to turn the huge amount abandon aluminum foil into recyclable sources for reproducing valuable electric materials” has become a meaningful study in the fields of energy, environment, and sustainable development. This study aims to study the reproducing aluminum nitride which combines its atoms by covalent bond and its structures is hexagonal crystal wurtzite, providing the high melting point and great thermal conductivity. Since aluminum nitride compounds have high thermal conductivity coefficient, electrical insulation and is a non-metal solid; and have similar low thermal expansion coefficient, low -mediated electric constant, low-mediated power loss to silicon has, so aluminum nitride is widely applied on microelectronic fields. Its thermal capacity is much higher than alumina, the thermal conductivity is no less than 7 times better than alumina. In Table 1 shown the structures and plate material properties of materials. The aluminum nitride can be seen with the superior thermal, electrical, mechanical characteristics, so the potential applications of aluminum nitride in thermal conductivity device are very large, as shown in Table 1. The outlook of aluminum nitride powder which made by put aluminum foil in a nitrogen ambient heated to 1270? for 4 hours is gray. The aluminum nitride powder added 1% Y2O3 through the re-grinding, particle, forming, and finally heated in a nitrogen ambient to 1500? sintering for 2 hours to form sintered substrate.
Analysis of aluminum nitride
Since aluminum nitride compounds high thermal conductivity coefficient, electrical insulation and a non-metal solid; which similar to the Silicon with low coefficient of expansion and the low dielectric constant, low dielectric loss, so aluminum nitride is widely applied on microelectronic fields. Its thermal capacity is much higher than alumina, the thermal conductivity is more than 7 times better than alumina. In Table 1 shown the structures and plate material properties of materials. The aluminum nitride can be seen with the superior thermal, electrical, mechanical characteristics, so the potential applications of aluminum nitride in thermal conductivity device are very large, as shown in Table 1.
|
Items of properties |
Preparation samples |
Commercial products |
Unit |
|
Theoretical density |
3.45 |
3.26 |
g/cm3 |
|
Structure |
Hexagonal |
Hexagonal |
-- |
|
Specific heat (25 oC) |
790 |
730 |
J/kg. K |
|
Thermal conductivity (25 oC) |
127.2 |
?160 |
W/m.K |
|
Resistance |
1.66*1012 |
1013?1014 |
W.m |
|
Permittivity (1MHz) |
7.9 |
8.6?9.0 |
-- |
Table 1: The structures and properties of aluminum nitride
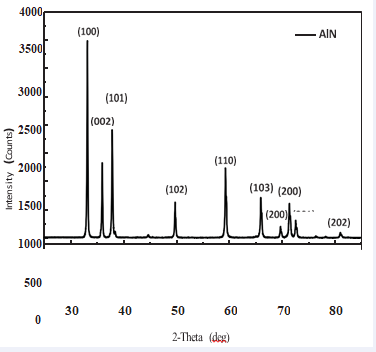
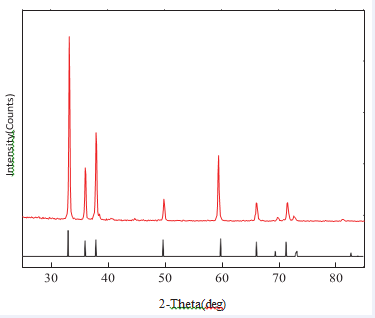
Aluminum nitride powder and the substrate crystal structure are analyzed by X-Ray Diffraction Analyzer (RIGAKU Multiflex), using Cu-Kα radiation, wavelength 1.5406 Å, operating voltage is 40 kV, operating current is 30 mA, measuring speed is 3 degree/Min. The 2θ scanning is 15°~ 85°, using JCPDS card as the material identification standard diffraction pattern. X-ray diffraction pattern contains many of crystal structure information, such as the type and size of the unit lattice can be defined by the diffraction peaks of the position (2θ), and the atom structure in the unit lattice can be defined by the relative position of the diffraction peak intensity. Figure 5, 6, respectively, shown the XRD pattern of the aluminum
Figure 5: XRD pattern of the aluminum nitride powder
Figure 6: XRD pattern of the aluminum nitride and yttrium oxide
substratenitride powder and the substrate. Diffraction angle at 33°, 36.1 °, 38 °, 50 °, 59 °, 66°, 70°, 72.7°, 73.5° and 82.0° ofthe diffraction peak can be seen same as the JCPDS (09- 0063) standard peak in the bottom. That’s meaning the product has single LiNiO2 layer-rock salt structure, and no impurity phase is produced.
Process of the aluminum nitride benefit analysis
The distinguishing feature of this process is using solvent-filter to separate aluminum foil first, then using aluminum foil to produce aluminum nitride. Because aluminum foil is made by rolling the aluminum ingots many
times. The production of aluminum ingots is an energy- consuming industry. It costs about 8 kWh to produce one kilogram of aluminum ingot. To make ingots into aluminum powder or aluminum foil is an energy-consuming industry. However, the nitridation of aluminum must have a huge surface area. Using ready-made scrap aluminum foil, nitridation it into aluminum nitride can save a lot of energy. Even if only solvent filtration separation, the waste aluminum foil separated into aluminum foil, paper, and PE resin, and then aluminum foil pressed into aluminum ingots, can also save a lot of energy. The making of paper is a highly polluted industry, and it also needed cut many trees as raw materials. Get papers from scrap aluminum foil for pulp can avoid of cutting lots of trees. And the making of PE resin is using crude oil as raw material, extracting PE from waste aluminum foil can also reduce crude oil cost.
Assuming the specific heat of the solvent is 0.5 (cal/ g?), it requires 10 times solvent to heat to 100 ? to dissolve the PE resin. The energy required for dissolution process is 0.5* (100-25) *10 = 375 (cal/g). Assuming that the PE resin’s heat content same as crude oil is 10,000 (cal/g), this also does not include the required energy to make crude oil become PE resin, that is, only needed less than 1/27 of energy consumption to make the resin. If we recycle the waste aseptic pack and waste aluminum foil in stay of directly burn, is a standard energy efficiency and carbon reduction process. Facing the energy crisis and the greenhouse effect today, this process should be worth for further development, and meaning are significance to energy, environmental and social sustainable development.
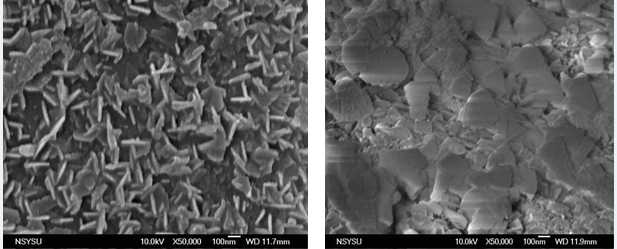
Figure 7: The SEM micro-structure of (a) Aluminum nitride powder and (b) Substrate
CONCLUSIONS
In this study, the method has been dissolved in the solvent separation of cardboard successfully, aluminum foil and a polyethylene resin powder from the waste aseptic package. Preparation of aluminum foil was placed under a nitrogen atmosphere and heated to 1270? nitriding treatment for 4 hours to obtain an aluminum nitride powder. Select the appropriate waste material for recycling is a very high economic efficiency technology, and solve the waste aseptic pack environmental pollution problems at the same time. The aluminum nitride powder through the re-grinding, particling, forming, and finally heated to 1500? in a nitrogen ambient sintering for 2 hours to form sintered aluminum nitride and yttrium oxide substrate. The results revealed that the aluminum nitride powders are uniform in the range of 100 nm, and the aluminum nitride can be seen with the superior thermal, electrical, mechanical characteristics. It has been widely used on photoelectric industry products.
ACKNOWLEDGMENTS
This work is grateful to professor C. H. Lin for critical reading and the authors thank Ministry of Education of ROC for financial support (100G-32-006).
REFERENCES
- C Giordano, I Ingrosso, MT Todaro, Maruccio S. De Guido, R. Cingolani, et al. AlN on polysilicon piezoelectric cantilevers for sensors/ actuators. Microelectron. 2009; 86: 1204-1207.
- Shyan-Lung Chung, Cheng-Yu Hsieh, Chih-Wei Chang. Enhancement of thermal conductivity in ceramics obtained from a combustion synthesized AIN powder by microwave sintering and reheating. J Mater Res. 2008; 23: 819-827.
- SL Chung, TI Tsai, SC Huang. High thermal conductivity ceramics from combustion synthesized AlN powder through microwave sintering and reheating. Int J Self-Propag High-Temp Synth. 2012; 21: 45-50.
- Chun-Nan Lin, Shyan-Lung Chung. A combustion synthesis method for synthesis of aluminum nitride powder using aluminum containers. J Mater Res. 2011; 16: 3518-3525.
- S.L. Chung, C.N. Lin, Z. X. Lin. Method for synthesis of aluminum nitride, US Patent. 2002; 6: 384.
- SH Xie, BK Zhu, JB Li, XZ Wei, ZK Xu. Preparation and properties of polyimide/aluminum nitride composites. Polym Test. 23; 7: 797-801.
- Yoshikazu Kameshima, Masaki Irie, Atsuo Yasumori, Kiyoshi Okada. Mechano-chemical effect on low temperature synthesis of AlN by direct nitridation method. Solid State Ion. 2004; 172: 185-190.
- Wei-Chang Lee, Chang-Lin Tu, Chang-Yueh Weng, Shyan-Lung Chung. A Novel process for combustion synthesis of AlN powder. J Mater Res. 2011; 10: 774-778.
- S. H. Hong, B. K. Kim. Fabrication of aluminum flake powder from foil scrap by a wet ball milling process. Mater Letters. 2001; 51: 139-143.
- Chun-Nan Lin, Shyan-Lung Chung. Combustion synthesis of aluminum nitride powder using additives. J Mater Res. 2001; 16: 2200-2208.
- Alexander Gromov, Alexander Ilyin, Alexander Ditts, Vereshchagin. Combustion of Al–Al2O3 mixtures in air. J Eur Ceram. 2005; 25: 1575-1579
- Alexander Gromov, Vladimir Vereshchagin. Study of aluminum nitride formation by superfine aluminum powder combustion in air. J Eur Ceram. 2004; 24: 2879-2884.
- Yoshinori Tokoi, Tsuneo Suzuki, Tadachika Nakayama, Hisayuki Suematsu, Futao Kaneko, Koichi Niihara. Synthesis of Aluminum Nitride Nanopowder with Particle Size Less than 10 nm by Pulsed Wire Discharge in Nitrogen Gas. J Appl Phys. 2010; 49: 1162-1168.
- Xinrui Xu, Hanrui Zhuang, Wenlan Li, Suying Xu,Baolin Zhang, Xiren Fu. Improving thermal conductivity of Sm2O3-doped AlN ceramics by changing sintering conditions. J Matr Sci Eng. 2003; 342: 104 -108.
- S M Olhero, P. Miranzo, JMF Ferreira. AlN ceramics processed by aqueous slip casting. J Mater Res. 2006; 21: 2460-2469.
- MC Liu, YC Liu. Reproduction of Al2O3 from waste aluminum foil by Combustion method, The International Conference on Safety & Security Management and Engineering Technology. WuFeng Institute of Technology. 2010; 383-386.
- RAV Raff, JB Allison. Polyethylene. New York, Interscience Publishers. 1956.