Pressure-Assisted 3D Printing of Customizable Ondansetron Tablets: An example of On Demand Fabrication of Personalized Medicines
- 1. Department of Pharmaceutical Sciences and Technology, Institute of Chemical Technology, India
- 2. Department of Biological Sciences and Biotechnology, Institute of Chemical Technology, India
Abstract
Personalized drug delivery has gained significant attention to reduce treatment-related adverse effects and improve therapeutic efficacy. Despite its promise, one of the key challenges in implementing personalized medicines is the rapid and precise manufacturing of individualized dosage forms. In this study, we have employed pressure-assisted microsyringe (PAM)-based 3D printing to fabricate immediate-release (IR) tablets of ondansetron (OND), with customizable doses ranging from 4 mg to 24 mg. A novel application of hydroxypropyl methylcellulose (HPMC K100LV), typically used in sustained-release formulations, was explored for immediate release (IR) tablet printing by modulating the infill density. Microcrystalline cellulose and mannitol were incorporated in the ink formulation to enhance printability, mechanical strength and stability, while ensuring a high drug loading and controlled disintegration. Rheological analysis of the HPMC-based ink demonstrated non-Newtonian, shear-thinning (pseudoplastic) behavior, along with favorable extrudability through the printing nozzle. The printed tablets were assessed for critical quality attributes, such as weight uniformity, friability, and thickness. Additionally, the impact of post processing drying methods on surface morphology, disintegration time, and dissolution behavior was systematically evaluated on the tablets. Dissolution testing confirmed that immediate drug release was successfully achieved using HPMC K100LV-based ink, highlighting its versatility in 3D-printed personalized dosage forms.
Keywords
• 3D printing
• Ondansetron
• Semi-solid extrusion
• Rheology
• Flexible dosing
• Drying optimization
Citation
Khule K, Ganatra P, Epili R, Jain R, Dandekar P. (2025) Pressure-Assisted 3D Printing of Customizable Ondansetron Tablets: An example of On-Demand Fabrication of Personalized Medicines. J Materials Applied Sci 6(1): 1017.
ABBREVIATIONS
3D: Three Dimension; SSE: Semisolid Extrusion; PAM: Pressure-assisted Micro-syringe; DSC: Differential Scanning Calorimetry; HCL: Hydrochloride; OND: Ondansetron Hydrochloride; HPMC: Hydroxy propyl methylcellulose; CMC: Carboxymethylcellulose; MCC: Microcrystalline Cellulose; FD: Freeze Dried; VD: Vacuum Dried; OD: Oven Dried; Disso: Dissolution; XRD: X-Ray Diffraction; PPM: Parts Per Million; mm: millimeter; nm: nanometer; min: Minute; h: Hour.
INTRODUCTION
The current trends in the pharmaceutical industry are aimed at meeting the ever-growing demands for customized drug dosing and personalized formulations to improve patient compliance and treatment outcomes.The traditional manufacturing processes, based on the “one-size-fits-all” approach, lacks the flexibility required to produce tailored treatment regimens specific to an individual patient’s needs. Addressing these issues has been a topic of research over the last decade, where 3D printing technology has emerged as a promising solution for on-demand manufacturing of dosage forms. The 3D printing technology offers a plethora of platforms for precision drug delivery and dose modification as per the patient’s requirements, if required, in a remote setup [1].Customized drug delivery approach has proven to be a boon in oncology products, where targeted and precise doses play a critical role in reducing the adverse effects associated with the treatment regimens and improving their therapeutic efficacy [2,3]. Chemotherapy and radiation-induced nausea and vomiting are inevitable.Usually, Ondansetron is prescribed as the first line of treatment, as a part of the treatment regimen during chemotherapy [4-6].Despite its high safety profile, this drug results in several common side-effects such as headache (experienced by 8-42% of the population), fever, constipation, malaise, xerostomia (5-17%), allergic reactions, and diarrhoea (2-5%) [7,8]. Acute myocardial ischemia and arrhythmia have also been reported in adults, especially those with risk factors that may prolong the QTc interval. Elongation of QTc interval also enhances concern for Torsades de Pointes and other arrhythmias [9,10]. Therefore, elderly patients or other at-risk groups are recommended to undergo ECG monitoring, along with administration of potassium and magnesium compounds by the USFDA [11]. R. Chandrakal et al., reported an ‘ondansetron induced fatal ventricular tachycardia’ in a fourteen-year-old female child [12]. Freedman et al., has discussed about the risk of cardiac arrhythmias during post-marketing analysis of ondansetron (OND) [13]. In patients with severe hepatic impairments, the clearance of OND was lowered, which resulted in an increase in its volume of distribution and the plasma half-life. Moreover, the doses required by children and geriatric patients can vary tremendously as compared to the doses required by adults [11,14]. The probable risks associated with these adverse effects render it important to administer OND in adequate and appropriate doses to the patients. This necessitates the design of personalized medications to cater to the requirements of every individual patient, depending on their age, gender and/ or medical history. The currently marketed OND dosage forms have varying quantities of this drug, ranging from 4 mg to 24 mg, viz. 4 mg, 8 mg, 16 mg, 24 mg. Although these administer four distinct doses, they fail to take into consideration the intra-individual variability associated with most medications [11,15].There are a few reports of personalized medications of OND, using different 3D printing techniques. Allaham et al. employed Selective Laser Sintering (SLS) 3D printing to fabricate orally disintegrating printlets (ODPs) of OND using two mannitol-based formulations. Cyclodextrins were included for taste masking. Both formulations disintegrated in under 15 seconds and released over 90% of OND within 5 minutes, showing comparable efficacy to a commercial product [16]. Ferreira et al., used FDM 3D printing to produce personalized pediatric doses of OND and esomeprazole in a hospital setting, enabling tailored treatments, especially for children undergoing chemotherapy. While the study did not address taste masking of OND—raising compliance concerns—it highlighted the potential for customized drug release and combination therapies to enhance outcomes in pediatric care [17].OND, as a fast-acting anti-emetic, requires rapid formulation methods. FDM involves time-consuming filament preparation, while SLS is costly, limiting their use in point-of-care (PoC) settings. In contrast, semi-solid extrusion (SSE), or pressure-assisted microsyringe (PAM) printing, offers a simpler alternative by using ready-to print pastes, eliminating intermediate steps. Unlike FDM or DPE, PAM accommodates a broader range of excipients and APIs in semi-solid form without thermal degradation, making it ideal for flexible, PoC pharmaceutical manufacturing [18]. Funk et al., reviewed polymers used in SSE (PAM) 3D printing for immediate-release dosage forms, highlighting the roles of polymethacrylates, cellulosics, vinyl derivatives, and PEG in influencing dissolution, strength, and printability. Cellulosic polymers like HPC and HPMC enhanced paste viscosity and tablet integrity. The method’s ease of semi-solid preparation supports decentralized, personalized dosing in hospitals and pharmacies, offering advantages over traditional manufacturing [19].In this study, we have evaluated immediate-release 3D-printed OND tablets produced via PAM extrusion as personalized dosage forms. A tailored ink of HPMC K100LV, microcrystalline cellulose, and mannitol was optimized for printability, stability, and rapid disintegration. Although HPMC is typically used in sustained-release forms, its application here enabled effective immediate release. By adjusting tablet geometry or infill density, OND doses from 4 mg to 24 mg were achieved without reformulation. The impact of drying methods on porosity and stability was also assessed to ensure consistent performance. This work advances personalized medicine through flexible, scalable 3D-printed IR tablets.
MATERIALS AND METHODS
Materials
Ondansetron Hydrochloride (OND) (Yarrow Chem Products, Mumbai, India) was used as the model drug. Hydroxypropylmethyl cellulose polymer (HPMC K4M and HPMC K100LV) was obtained as gift samples from Colorcon (Harleysville, United States) and was chosen as a hydrophilic matrix with immediate release behavior. Avicel PH-101 and Sodium CMC were obtained as a gift sample from Signet (Mumbai, India). Mannitol Pearlitol 300 DC was purchased from S.A. Pharmachem (Mumbai, India). Croscarmellose sodium was obtained as a gift sample from DuPont (Mumbai, India).
Methods
Preparation of the 3D printing ink formulation:
Different placebo trials were conducted for the preliminary screening of polymers that could be processed into printable pastes, thereby allowing for precise deposition and layering during the printing process. The compositions of the printable inks have been listed in Table 1.
Table 1: Compositions of inks for placebo tablets.
|
Sr No. |
Ingredients |
Quantity in percentage (%) |
||||||
|
F1 |
F2 |
F3 |
F4 |
F9 |
F5 |
F6 |
||
|
1 |
Hydroxypropyl methyl Cellulose (HPMC K4M) |
11 |
6 |
6 |
6 |
6 |
2 |
4 |
|
2 |
Sodium CMC |
- |
16 |
- |
- |
- |
- |
- |
|
3 |
Microcrystalline Cellulose (Avicel PH 101) |
- |
- |
16 |
21 |
24 |
21 |
21 |
|
4 |
Water |
89 |
78 |
78 |
73 |
70 |
77 |
75 |
After assessing the feasibility of bio-ink composition, further trials were executed with OND API, as stated in Table 2, to investigate the influence of the bio-ink composition on the dissolution behavior. Microcrystalline Cellulose (Avicel PH 101), was used as a bulking agent to impart good mechanical properties; however, the poor disintegration associated with it led to incomplete drug release. Thus, mannitol was added as a pore former and filler, aiding in drug release from the tablets. Thus, equal proportions of both fillers were evaluated for the printing feasibility, since only mannitol possesses lower mechanical integrity after drying. The concentration of the conventional disintegrating agent, croscarmellose sodium, was fixed at 3%, and the infill density was altered to attain the desired release profile.
Table 2: Compositions of inks for the tablets with OND API.
|
Sr. No. |
Ingredients |
Quantity in percentage (%) |
|||
|
F13/F14 |
F15 |
F17 |
F19 |
||
|
1 |
Hydroxy propyl methyl Cellulose (HPMC K4M) |
3 |
1.5 |
- |
- |
|
2 |
Hydroxy propyl methyl Cellulose (HPMC K100LV) |
- |
1.5 |
3 |
3 |
|
3 |
Microcrystalline Cellulose (Avicel PH 101) |
21 |
21 |
21 |
18 |
|
4 |
Mannitol (Pearlitol 300 DC) |
21 |
21 |
21 |
21 |
|
5 |
Croscarmellose sodium (Ac-di-sol) |
- |
- |
- |
3 |
|
6 |
Purified Water |
50 |
50 |
50 |
50 |
|
7 |
Ondansetron HCL (OND) |
5 |
5 |
5 |
5 |
The printing ink was formulated by dispersing hydroxypropyl methylcellulose (3 gm) in purified water (50 ml) under continuous stirring, followed by a hydration period of 1 h to allow complete swelling and gel formation. The resulting gel was transferred to a mortar. OND, previously sieved through a #40 mesh, was gradually incorporated into the gel with constant trituration to ensure uniform dispersion. Subsequently, microcrystalline cellulose and mannitol were added incrementally with continuous mixing to obtain a homogeneous and viscous paste. The final formulation was then loaded into syringes for further use in the printing process.
Rheology of bioink formulation: The rheological properties of the optimized ink formulations (F14, F15, and F19) were assessed using a rotational rheometer (Anton Paar MCR301, Graz, Austria), equipped with a 25 mm parallel plate geometry. The plate gap was maintained at 1 mm throughout the measurements, and the temperature was controlled at 25?°C. Shear viscosity and the corresponding shear stress were recorded across a shear rate (range of 0.01 to 100 s?¹. Zero-shear viscosity (n) was determined by fitting the flow curve data to the Power law model equation 1
τ = K(dυ/dy)^n ….Equation 1
where,
τ (Shear Stress): Force per unit area required to deform the fluid.
dυ/dy (Shear Rate): Rate of change of velocity with respect to distance, indicating the deformation rate of the fluid.
K (Consistency Index): Measure of the fluid’s resistance to flow, often expressed in Pa·s^n.
n (Flow Behavior Index): Dimensionless parameter that determines the fluid’s behavior:
n = 1: Newtonian fluid (viscosity is constant).
0 < n < 1: Shear-thinning (pseudoplastic) fluid (viscosity decreases with increasing shear rate).
n > 1: Shear-thickening (dilatant) fluid (viscosity increases with increasing shear rate)
A three-interval thixotropy test (3iTT) was employed to simulate the extrusion and recovery behaviours during printing. The test involved application of (i) a low shear rate of 1 s?¹ for 60 s, (ii) a high shear rate of 30 s?¹ for 30 s, and (iii) a return to 1 s?¹ for 60 s to monitor structural recovery. The percentage recovery was computed as the ratio of the average viscosity during the third interval to that of the first interval [20,21].
Printing of tablets: Computer-aided design (CAD) software Fusion 360 (Autodesk, USA) was used to design the tablet templates, which were then exported to the 3D printer’s slicing software Mito (Avay Bioscience, Chennai, India) for further slicing. Different infill patterns (15%, 20% and 40%) were explored for printing optimization and drug loading.
Weight volume correlation: 3D printing enables volume-based, personalized dosing, but tablet dimensions may not directly reflect the dose due to ink density variations. Hence, a weight-to-volume correlation was established. Tablets with varying weights and infill densities (40%, 20%, 15%) were printed using paste F19 [Table 3]. After drying, tablet weights were plotted against their theoretical volumes to assess correlation [20,21].
Table 3: Infill percentages for 3D printing.
|
Sr. No. |
Infill percentage |
Tablet Weight (mg) |
Dose (mg) |
|
1 |
40% |
40 mg |
4 mg |
|
2 |
20% |
80 mg |
8 mg |
|
3 |
20% |
160 mg |
16 mg |
|
4 |
15% |
240 mg |
24 mg |
Drying of the 3D printed tablets: Three different types of drying processes, viz, oven drying, vacuum drying, and freeze drying, were evaluated to achieve the best resolution for the printed tablets. The drying parameters have been listed in Table 4.
Table 4: Drying parameters for post-processing of 3D-printed tablets
|
Oven Drying |
Freeze drying |
Vaccuum Drying |
|
Hot Air Oven |
Lyophilizer |
Buchi Rotavapor R-300 (Flawil, Switzerland) |
|
At 60? for 12 h |
Initially freezed at -80°C for 10 h and then dried at a condenser temperature of -39°C and a pressure of -65 mBar for 24 h |
At 40? for 12 h – 15 h |
Physico-chemical evaluation of 3D printed tablets: Unlike the conventional compression-based manufacturing, the PAM 3D printing method involved hydrating the binder and filler, which resulted in dried tablets after extrusion. In such cases, the tablets should comply with the United States Pharmacopeia (USP) standards, regarding their quality and physical attributes. In the present work, we evaluated 3D-printed tablets for their hardness, friability, weight variation, crystallinity, and porosity.
Weight variation test: Twenty (20) tablets were randomly selected and individually weighed, using a weighing balance (CITIZEN, Mumbai, India). The average tablet weight and percentage deviation for each tablet were determined.
Friability test: Friability was tested using a Roche friabilator (FT1020, LABINDIA) with 10 tablets rotated at 25 rpm for 4 minutes (100 rotations). Tablets were weighed before and after the test, and percent friability was calculated using Equation 2. A weight loss ≤1.0% was considered acceptable.
Hardness Test: Tablet hardness was measured using a Monsanto hardness tester. Six tablets from the optimized batch were tested by applying pressure until breakage, and the breaking force (kg/cm²) was recorded as the difference between initial and final readings.
In-vitro disintegration test: In- vitro drug disintegration test was carried out using a tablet disintegration apparatus (Electrolab, India). The tablets were placed in the disintegration apparatus, at a temperature of 37 ± 2 °C, in 900 mL of water. Time required for disintegration of tablet was observed and noted (n= 3).
Drug content: Drug content was determined using six tablets of each type (10% loading; 40–240 mg). Tablets were dissolved in water in 100 mL volumetric flasks using magnetic stirring (60 min, 250 rpm) and sonication (10 min). The volume was adjusted to 100 mL, then diluted to 10?µg/mL and analyzed by UV-Vis spectroscopy. All measurements were done in triplicate.
In vitro drug release (Dissolution test): An in vitro dissolution test was performed using USP Type II (Paddle) apparatus (Electrolab, Mumbai. India). The details of the protocol have been stated in Table 5. At each sampling point, aliquots of 5 mL were withdrawn, and this volume was replaced with fresh medium. The aliquots were quantified using UV-Visible spectroscopy at 248 nm.
Table 5: In Vitro dissolution protocol for 3D printed table
|
Apparatus |
USP II (Paddle) (Electro lab India) |
|
Medium |
Water |
|
Volume |
900 mL |
|
Temperature |
37 ± 0.5 °C |
|
Speed |
50 rpm |
|
Sample Volume |
5 mL |
|
Sampling point |
15, 30, 45 min |
|
Λmax |
248 nm |
Surface roughness measurement of 3D printed tablets: For 3D printed tablets, surface roughness plays a key role during dissolution. LEXT (OLS5000) measuring laser microscope was used for the measurement of surface roughness. Oven-dried, freeze-dried, and vacuum-dried 3D-printed tablets were placed under the microscope for analysis. The parameters were set as stated in Table 6.
Table 6: Surface roughness evaluation parameters for the laser microscope.
|
Sr. No. |
Measurement condition |
Details |
|
1 |
Objective Lens |
MPLAPON50xLEXT |
|
2 |
System Name |
OLS5000-SAF |
|
3 |
Scanning mode |
3D Standard + Color |
|
4 |
Zoom |
1x |
|
5 |
Lase Brightness |
63.4 |
|
6 |
Laser Intensity |
100 |
RESULTS AND DISCUSSION
Extrusion and printability of formulated paste
In the pressure-assisted microsyringe (PAM) 3D printing process, bio-ink (paste) plays a critical role in ensuring easy extrusion, structural integrity, and mechanical strength of the printed tablets. The ink’s composition, particularly the concentration of materials used, directly affects its extrudability, buildability, viscosity, and overall quality of the printed product [22]. HPMC has been extensively used as a binder in tablets and as a hydrophilic matrix for controlled drug release. However, two different HPMC grades were explored in this study for achieving an immediate release profile, when combined with superdisintegrants, and printing tablets with varying infill densities, thereby allowing the drug release process to be suitably tailored [23,24].
First, the impact of the amount of HPMC used during paste preparation was assessed on the characteristics of the resulting tablets. PAM 3D printing pastes are generally composed of hydrogels, and thus, HPMC hydrogel would affect the properties of the 3D-printed tablets [25]. Batch F1, which consisted of only HPMC K4M (11%), resulted in a watery, gel-like consistency due to HPMC’s hydrophilic nature and its ability to absorb water. Additionally, it also lacked sufficient viscosity for optimum printing. Thus, it was hypothesized that combining HPMC K4M (6%) with sodium CMC (16%) (F2) would improve the viscosity and could provide a suitable matrix for drug release [26,27]. However, the combination resulted in a highly viscous paste that was not suitable for extrusion-based 3D printing, as evidenced by the excessive extrusion pressure observed in Table 7
Table 7: Pressure observed during placebo trials.
|
Parameter |
Quantity in percentage (%) |
||||||
|
F1 |
F2 |
F3 |
F4 |
F9 |
F5 |
F6 |
|
|
Pressure (mPa) |
0.27 |
0.325 |
0.162 |
0.209 |
0.26 |
0.08 |
0.122 |
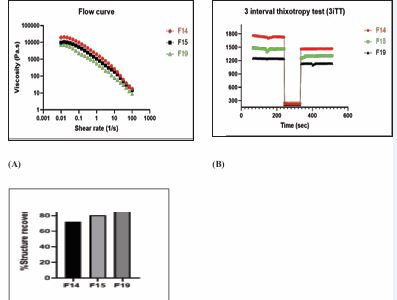
Hence, microcrystalline cellulose (16%) was introduced into the formulation (F3) to improve the consistency of the paste prepared using HPMC K4M (6%) [28]. Different concentrations of microcrystalline cellulose (16% and 21%) (F3 and F4) were evaluated to assess its impact on the structural rigidity of the formulation. Further, increased concentration of MCC (24%) (F9) acted as a bulking agent and binder, contributing to the mechanical strength and densification of the tablet. Also, reduction in HPMC K4M concentration (2% and 4%) (F4 and F6), when MCC content was kept constant, further optimized the bio-ink for the printing process [29]. It was then observed that the decrease in HPMC content reduced the viscosity, improving flow during printing. However, the higher fluidity of the printer ink (2%) (F5) resulted in the fluid partially collapsing under its own weight during the drying process [29,30]. Hence, further trials were conducted with OND at the HPMC concentration of 3% [Table 2]. The printing process was conducted smoothly, while MCC enhanced the ink’s structural integrity, preventing deformation during the process [31]. However, the tablets were not able to disintegrate immediately, thus requiring the inclusion of mannitol as the filler and pore former. The addition of mannitol (F15, F16, and F17) improved mechanical properties, accelerated disintegration, and modulated drug release [32,33]. Mannitol’s ability to form micropores and dissolve rapidly led to faster dissolution rates, preventing structural collapse of tablets during drying.The developed ink was able to yield formulation with immediate release (F13/F14), however required high pressure during the printing process, as indicated in Table 8. It was hypothesized that it could be due to the high viscosity grade of HPMC K4M. This was confirmed when the lower viscosity grade of HPMC K100 LV was combined with the higher viscosity grade of HPMC K4M in equal proportion (F15), which enabled easier printing at a lower pressure compared to formulations containing only HPMC K4M (F13/F14). Moreover, it also aided in modulating the drug release profile from the tablets. However, favorable dissolution results and processing ease were obtained when HPMC K100 LV (F17 and F19) was used as the only polymer for the fabrication of 3D-printed tablets [30,34]. Batch F19 was identified as the most promising for producing 3D-printed tablets with immediate release properties due to the presence of superdisintegrant croscarmellose sodium, demonstrating the importance of fine-tuning ink formulations to achieve desired printing characteristics and tablet performance.In the context of semi-solid extrusion (SSE) 3D printing, the rheological behavior of the ink plays a pivotal role in determining its printability and structural performance. Our results highlighted the importance of achieving a balance between the viscous and elastic properties of the ink, under dynamic conditions. An ideal ink formulation must exhibit a sufficiently high yield stress to prevent unintended flow within the cartridge, pronounced shear thinning behavior to enable smooth extrusion under applied pressure, and rapid elastic recovery post-extrusion to maintain the structural integrity and shape fidelity of the printed constructs [20,35]. These characteristics are essential for ensuring consistent layer deposition and dimensional accuracy during the printing process. In this study, three different concentrations of polymers (HPMC K4M, HPMC K4M: HPMC K100LV and HPMC K100LV) were used to investigate the flow behavior in different batches. Formulation batches F14, F15 and F19 were studied to evaluate their flow behavior and structural recovery after extrusion. Figure 1 depicts the rheological behavior of the inks from batches F14, F15 and F19. Figure 1 (A) represents the viscoelastic flow curve of all the pastes that were studied. It was observed that the viscosity of the formulations drastically decreased for all the batches with an increase in shear rate. This was also seen from the n values (flow indices) stated in Table 9.
|
Batch no |
Flow Behaviour (n) |
Consistency Index (K, Pa·s?) |
R² |
Interpretation |
|
F14 |
0.223 |
6643.16 |
0.488 |
Shear-thinning, weak power-law fit |
|
F15 |
0.326 |
7038.20 |
0.686 |
Shear-thinning, better fit |
|
F19 |
0.325 |
3144.64 |
0.816 |
Shear-thinning, best power-law fit |
A gel or paste is considered shear thinning when n < 1. Polamaplly et al., reported that the HPMC gel exhibits shear-thinning properties [36,37]. When soluble and insoluble diluents are suspended in a HPMC gel matrix, particle-particle interaction increases. If the paste is saturated with the solid content, this particle-particle interaction increases manifold, and thereby, the non-Newtonian behavior becomes prominent [38]. This characteristic allows the paste to extrude through a fine nozzle when air pressure is applied.As observed from Table 9, a higher K value indicated the materials’ resistance to flow, thereby demonstrating that F19 ink exhibited the lowest resistance to flow, which was desired for a smooth printing process. Moreover, R2 value of 0.816 also depicted that this was the best fit in the model, and that F19 ink had the optimum shear-thinning property. Additionally, the insights provided by 3iTT data presented in Figure 1 (B) also supported that F15 and F19 showed superior structural recovery compared to F14, making them more suitable for applications requiring rapid.
Figure 1 (A) - Flow curve (Viscosity vs Shear rate), (B) - 3 Interval Thixotropy Test (C) - Structural Recovery of ink formulations of batch F14, F15, and F19
reformation post-shear, such as in extrusion-based drug delivery systems. Moreover, it was observed that when the polymer grade was changed from HPMC K4M (F14) to HPMC K100LV (F19), while keeping the concentration constant, the percent recovery [Figure 1 (C)] considerably increased, which demonstrated that HPMC K100 LV (F19) could be used for smooth on-demand printing of tablets in a remote setup.After evaluating the rheological characteristics for selecting ink composition, it was essential to evaluate the capability of PAM-based 3D printing for personalized dosing of OND. Tablets with varying dimensions and infill densities were fabricated to develop a calibration curve, correlating apparent volume with tablet weight. The apparent volume was calculated from the tablet’s dimensions (length × width × height), and weight was used as a surrogate for drug dose, as depicted in Table 10 and Figure 2 (A) (B) (C) (D), assuming a uniform drug loading. This demonstrated the accuracy and predictability of the method in tailoring tablet weight—and consequently drug dose—based on design parameters.
Table 10: Printing Metrics for tablet weight & dose.
|
Sr. No. |
Selected infill % |
Dimensions (mm) |
Dose (mg) |
Weight (mg) |
|
1 |
40 |
6*3*3 |
4 |
40 |
|
2 |
20 |
10*5*4.5 |
8 |
80 |
|
3 |
20 |
16*8*4 |
16 |
160 |
|
4 |
15 |
18*9*4.5 |
24 |
240 |
Figure 2: Varying dimension of tablets (length * width * height). (A) - 6*3*3, (B) - 10*5*4.5, (C) -16*8*4, (D) - 18*9*4.5
Figure 3 Calibration curve for dose and dimension correlation. (A) - 40% infill density, (B) - 20% infill density, (C) - 15% infill density.
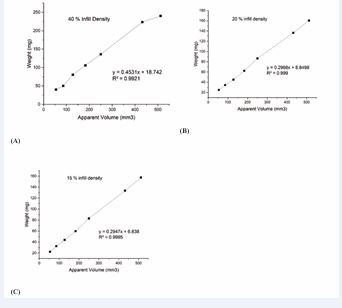
Table 11 to 13 and Figures 3A to 3C depicts the data and calibration curves for each infill density. At a constant volume, increasing the infill percentage led to a proportional increase in tablet weight [39-41]. For example, a tablet with a volume of 432 mm³ weighed 223.5 mg at 40% infill, 136.43 mg at 20%, and 133.5 mg at 15%. These results validated that dose modulation could be achieved by adjusting the infill density, without altering the ink formulation. The selected infill percentages also aligned with specific dosing requirements for OND, ranging from 4 mg to 24 mg, as shown in Table 10. This strategy enabled precise and flexible dose customization, essential for patient-specific therapies.
Table 11: Apparent volume (mm3) vs weight in mg for 40% infill density.
|
Sr. No. |
Apparent Volume (mm3) |
Dimensions (mm) |
Weight (mg) |
|
1 |
54 |
6*3*3 |
40.16 |
|
2 |
85.75 |
7*3.5*3.5 |
50.12 |
|
3 |
128 |
8*4*4 |
80.33 |
|
4 |
183 |
9*4.5*4.5 |
105.9 |
|
5 |
250 |
10*5*4.5 |
136.21 |
|
6 |
432 |
12*6*6 |
223.5 |
|
7 |
512 |
16*8*4 |
240.28 |
Table 13: Apparent volume(mm3) vs weight in mg for 15% infill density
|
Sr. No. |
Apparent Volume (mm3) |
Dimensions (mm) |
Weight (mg) |
|
1 |
54 |
6*3*3 |
22.3 |
|
2 |
85.75 |
7*3.5*3.5 |
32.5 |
|
3 |
128 |
8*4*4 |
43.9 |
|
4 |
183 |
9*4.5*4.5 |
59.8 |
|
5 |
250 |
10*5*5 |
83 |
|
6 |
432 |
12*6*6 |
133.5 |
|
7 |
512 |
16*8*4 |
157.5 |
Overall, the PAM 3D printing approach demonstrated strong linearity and reproducibility in establishing a relationship between tablet geometry, infill density, and weight. These results confirmed its potential as a reliable method for producing personalized oral dosage forms, with a high accuracy.
Post-processing process optimization
Post-processing plays a critical role in improving the physical and mechanical properties of semi-solid extrusion (SSE) 3D-printed tablets. For moisture-rich formulations such as hydrogels or pastes, drying is essential to enhance stability, structural integrity, and drug release characteristics. In this study, three drying techniques, viz. oven drying, vacuum drying, and freeze drying, were assessed using tablets from batch F19 [42]. Among these, freeze drying was observed as the most effective method, with respect to preserving tablet geometry, preventing deformation, and maintaining the structural integrity of the tablet. In contrast, oven drying resulted in significant shape distortion and shrinkage, while vacuum drying showed an intermediate performance, with partial collapse in some cases. The outcomes highlighted in Table 14, demonstrate the limitations of conventional 40 80 160 240 Weight (mg) 40.16 50.12 80.33 105.9 136.21 223.5 240.28 drying methods and reinforce the importance of adopting optimized approaches for pharmaceutical 3D printing.
Table 14: Post-processing observations for different drying strategies.
|
Drying Method |
Oven Drying |
Freeze Drying |
Vacuum Drying |
|
Observation |
Considerable shrinkage and deformation, resulting in tablets with a film-like appearance |
Exhibited superior shape retention by preventing collapse or shrinkage, and ensuring structural integrity compared to those dried with the oven or vacuum methods. |
Moderate performance, with some shape distortion observed, |
|
Reason |
High drying temperatures caused rapid moisture loss and material collapse. |
The low-temperature process solidified the matrix, preserving its integrity throughout the drying process. |
Due to the insufficient control over temperature and pressure, which lead to partial collapse or shrinkage |
|
Roughness Average (μm) |
849.973 |
1112.20 |
972.52 |
|
|
Roughness Root Mean Square(μm) |
963.224 |
1275.97 |
1074.70 |
|
|
Laser Microscope Surface Roughness image |
|
|
|
|
|
|
|
|
||
|
3D image |
|
|
|
Freeze drying’s low-temperature and pressure controlled environment minimized thermal degradation and enabled the solidification of the tablet matrix during the drying phase [43]. Additionally, freeze-dried tablets exhibited higher porosity, which was advantageous for enhancing drug release rates [44]. Surface roughness analysis further supported these findings, as seen from Table 14 and Figure 4. Freeze-dried tablets demonstrated the highest surface roughness values (Ra = 1112.20 μm, Rq = 1275.97 μm) [Table 14 and Figure 4A], followed by vacuum drying (Ra = 972.52 μm, Rq = 1074.70 μm) (Table14 and Figure 4B, and oven drying (Ra = 849.97 μm, Rq = 963.22 μm) [Table 14 and Figure 4C], indicating a more porous and potentially faster-dissolving structure.Thus, freeze drying was found to be the most suitable post-processing method for PAM 3D-printed tablets, offering superior shape retention, structural fidelity, and surface characteristics conducive to enhanced drug release.
Physico-chemical evaluation of the printed tablets
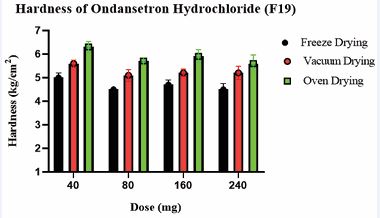
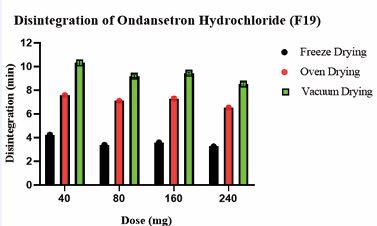
Comparatively less weight variation was observed for freeze-dried tablets compared to vacuum and oven-dried tablets. This was attributed to the robust nature of the freeze-drying process. Due to the highly porous nature of freeze-dried tablets, the hardness of freeze-dried tablets was comparatively lower [45,46]. However, they met USP standards, indicating adequate structural integrity across all tablet formulations. The hardness of the OND tablets has been summarized in Table 15 and illustrated in Figure 5. The % weight loss of 3D printed tablets (F19) for freeze dried, oven-dried, and vacuum-dried formulations was below 1%, which complied with the USP standards and confirmed their stability and structural integrity. Freeze dried tablets (F19) demonstrated significantly faster disintegration (3.25–4.23 min) compared to vacuum-dried and oven-dried tablets (6.53–10.30 min), possibly due to their higher porosity. The disintegration times of the OND tablets have been summarized in Table 15 and illustrated in Figure 6.
Table 15: Physical characterization for 3D printed tablets for optimized batch no F19
|
Drying Method |
Dose (mg) |
Weight Variations (mg) |
Hardness (kg/cm2) |
Friability (%loss) |
Disintegration (min) |
|
Freeze Drying |
4 |
40.05±0.98 |
5±0.2 |
0.30±0.015 |
4.23±0.15 |
|
8 |
79.58±0.90 |
4.5±0.0.30 |
0.38±0.036 |
3.38±0.065 |
|
|
16 |
159.645±0.94 |
4.7±0.2 |
0.48±0.036 |
3.58±0.017 |
|
|
24 |
239.635±0.81 |
4.5±0.25 |
0.71±0.023 |
3.25±0.025 |
|
|
Oven Drying |
4 |
39.16±2.50 |
6.3±0.23 |
0.35±0.01 |
10.30±0.22 |
|
8 |
78.65±2.39 |
5.7±0.15 |
0.39±0.04 |
9.20±0.12 |
|
|
16 |
158.74±2.31 |
5.90±0.28 |
0.47±0.06 |
9.45±0.098 |
|
|
24 |
238.81±210 |
5.60±0.36 |
0.62±0.08 |
8.51±0.18 |
|
|
Vacuum Drying |
4 |
39.67±1.90 |
5.6±0.15 |
0.34±0.04 |
7.58±0.12 |
|
8 |
79.58±1.10. |
5.10±0.25 |
0.42±0.03 |
7.10±0.09 |
|
|
16 |
159.6±1.03 |
5.20±0.16 |
0.51±0.02 |
7.29±0.18 |
|
|
24 |
239.5±0.93 |
5.20±0.28 |
0.65±0.07 |
6.53±0.098 |
Figure 5 Comparative graph depicts the impact of the drying process across all strengths on the hardness of tablets
Figure 6 Comparative graph depicts impact of the drying process across all strengths on the disintegration of tablets
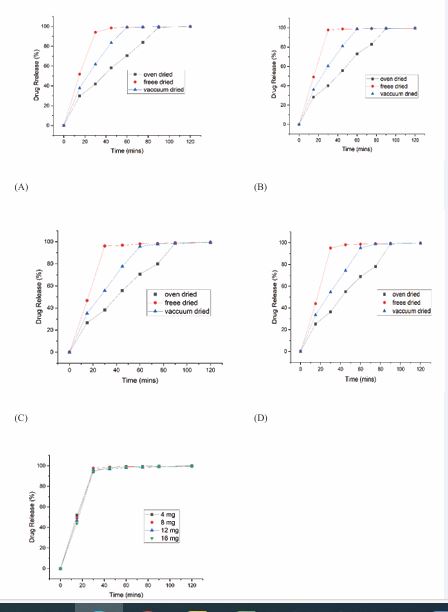
Various processing factors were optimized based on drug release from the printed tablets. It was observed from Figure 7A, 7B, 7C and 7D, that the percent cumulative drug release from freeze-dried, vacuum-dried, and oven dried OND tablets (F19) varied significantly, with freeze dried tablets exhibiting superior drug release [47]. The difference in drug release between freeze-dried batches.
Figure 7:
(A)- In vitro drug release of Batch 19 with 4 mg dose and 40% infill density
(B)- In vitro drug release of Batch 19 with 8 mg dose and 20% infill density
(C)- In vitro drug release of Batch 19 with 16 mg dose and 20% infill density
(D)- In vitro drug release of Batch 19 with 24 mg dose and 15% infill density
(E)- In vitro drug release of Batch F19 with 4 mg, 8 mg,16 mg, 24 mg of drug and optimized infill density
(F13 and F14) as mentioned in Table 16, was attributed to variations in infill density, with higher infill density (F13) resulting in lower drug release, due to reduced porosity and a more compact structure [48,49]. The optimum ink formulation, F19, with tablets weighing 40 mg to 240 mg, met the immediate-release.
|
Batch. No. |
Dimensions (mm)e |
Tablet Weight (mg) |
Weight Variation |
Time (minutes) |
% Cumulative drug release |
Drug content (%) |
|
|
F13 |
(60%Infill) 8*8*4 |
250 |
249.43±1.52 |
30 |
27.76±0.80 |
99±1 |
|
|
F14 |
(40%Infill) 6*3*3 |
40 |
39.83±1.09 |
30 |
81.1±0.94 |
98.50±0.50 |
|
|
(40%Infill) 8*4*4 |
80 |
79.53± 1.11 |
30 |
55.6±0.35 |
99.10+0.2 |
||
|
(40%Infill) 15.8*7.9*3.6 |
250 |
250.5± 0.84 |
30 |
31.1±0.70 |
99.60+1 |
criteria, achieving >90% drug release within 30 min [Figure 7 E]. This rapid release was facilitated by the hydrophilic properties of HPMC K100 LV, which promoted disintegration and dissolution, ensuring consistent and fast drug release. In contrast, vacuum-dried and oven dried tablets showed slower drug release, with over 80% released in 45 min and 75 mins, respectively [Figure 7A, 7B, 7C and 7D]. The superior release from freeze-dried tablets was attributed to their higher porosity, which allowed for faster dissolution [47].Overall, these findings underscore the critical influence of drying technique and infill density on release kinetics. The rapid disintegration and dissolution of the freeze dried F19 tablets, driven by the hydrophilic nature of HPMC K100 LV, highlight the formulation’s potential to offer a fast-acting, patient-compliant alternative superior to conventional marketed OND products.
CONCLUSION
Semisolid extrusion-based 3D printing was successfully employed for the fabrication of immediate-release OND tablets. The developed ink demonstrated a linear correlation between tablet mass and theoretical volume, confirming that dimensional adjustments effectively resulted in precise dosing. Additionally, the reduction in infill density was found to modulate the drug release profile from the 3D-printed tablets. Freeze-dried tablets exhibited faster drug release compared to vacuum or oven dried products, likely due to their lower porosity. The developed formulations resulted in fast disintegration (~15 s) and released more than 90% of the drug in 5 min, independent of the strength and tablet dimension. This technology was able to generate high-quality pharmaceutical products with an on-demand dose dispensing feature, to tailor tablet dimensions as per the therapeutic requirements. Future work can focus on refining the process and exploring its application for other APIs, paving the way for broader commercialization and clinical adoption.
ACKNOWLEDGEMENTS
The authors are thankful to DSTC-PURSE (PURSE program 2020; TPN-57255) for laboratory instruments and infrastructure. K.G is thankful to AICTE for the fellowship. P.G. is thankful to PMRF and BIRAC for providing funds to execute the project and for providing a fellowship. R.E is thankful to the Department of Science and Technology, Government of India (DST/TDT/AM/2022/281) for the fellowship.
CRediT authorship contribution statement
Krishna Khule: Conceptualization, Methodology, Investigation, Visualization; Conceptualization, Pankti Methodology, Ganatra: Investigation, Visualization; Rashmita Epili: Visualization, Writing - original draft; Ratnesh Jain: Conceptualization, Supervision, Resources; Prajakta Dandekar: Conceptualization, Supervision, Resources, Writing - review & amp; editing.
REFERENCES
- Elkasabgy NA, Mahmoud AA, Maged A. 3D printing: An appealing route for customized drug delivery systems. Int J Pharm. 2020; 588: 119732.
- Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M, et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). 2018; 6: 79-100.
- Su J, Yang L, Sun Z, Zhan X. Personalized Drug Therapy: Innovative Concept Guided With Proteoformics. Mol Cell Proteomics. 2024; 23: 100737.
- Eloise Neumann, Jason Patel. GUIDELINE FOR THE MANAGEMENT OF CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING. 2016.
- Antiemetic Guidelines for Chemotherapy and Radiation Therapy.
- Ashour AM. The preventive effects of ondansetron on chemotherapy- induced nausea and vomiting in adult cancer patients: systematic review from ClinicalTrials.gov. Front Pharmacol. 2023; 14: 1310455.
- Khan RB. Migraine-type headaches in children receiving chemotherapy and ondansetron. J Child Neurol. 2002; 17: 857-858.
- Christofaki M, Papaioannou A. Ondansetron: a review of pharmacokinetics and clinical experience in postoperative nausea and vomiting. Expert Opin Drug Metab Toxicol. 2014; 10: 437-444.
- Patel E, Rosemond D, Afzal A. Ondansetron induced torsades depointes. Clin Case Rep. 2019; 7: 1557-1558.
- Singh K, Jain A, Panchal I, Madan H, Gupta A, Sharma A. Ondansetron- induced QT prolongation among various age groups: a systematic review and meta-analysis. Egypt Heart J. 2023; 75: 56.
- Griddine A, Bush JS. Ondansetron. 2025.
- Chandrakala R, Vijayashankara CN, Kumar KK, Sarala N. Ondansetron induced fatal ventricular tachycardia. Indian J Pharmacol. 2008; 40: 186-187.
- Freedman SB, Uleryk E, Rumantir M, Finkelstein Y. Ondansetron and the risk of cardiac arrhythmias: a systematic review and postmarketing analysis. Ann Emerg Med. 2014; 64: 19-25.e6.
- Figg WD, Dukes GE, Pritchard JF, Hermann DJ, Lesesne HR, CarsonSW, et al. Pharmacokinetics of ondansetron in patients with hepatic insufficiency. J Clin Pharmacol. 1996; 36: 206-215.
- Christian G. Daughton, Ilene Sue Ruhoy. Lower-dose prescribing: Minimizing “side effects” of pharmaceuticals on society and the environment. Science of The Total Environment. 2013; 443: 324-337.
- Allahham N, Fina F, Marcuta C, Kraschew L, Mohr W, Gaisford S, et al. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics. 2020; 12: 110.
- Ferreira Mariana, Lopes Carla Martins, Gonçalves Hugo, Pinto João F, Catita José. Personalised Esomeprazole and Ondansetron 3D Printing Formulations in Hospital Paediatric Environment: I-Pre-Formulation Studies. Applied Sciences. 2022; 12: 10585.
- Peng H, Han B, Tong T, Jin X, Peng Y, Guo M, et al. 3D printing processes in precise drug delivery for personalized medicine. Biofabrication. 2024; 16: 10.1088/1758-5090/ad3a14
- Funk NL, Fantaus S, Beck RCR. Immediate release 3D printed oral dosage forms: How different polymers have been explored to reach suitable drug release behaviour. Int J Pharm. 2022; 625: 122066.
- Ganatra P, Jyothish L, Mahankal V, Sawant T, Dandekar P, Jain R. Drug- loaded vegan gummies for personalized dosing of simethicone: A feasibility study of semi-solid extrusion-based 3D printing of pectin- based low-calorie drug gummies. Int J Pharm. 2024; 651: 123777.
- Ganatra P, Ashapogu A, Epili R, Dugam S, Desai J, Jain R, et al. Biomimetic replenishment therapy of cortisol using semi-solid extrusion - 3D printed tablets for adrenal insufficiencies. Int J Pharm. 2025; 672: 125342.
- Khaled SA, Alexander MR, Wildman RD, Wallace MJ, Sharpe S, Yoo J, et al. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int J Pharm. 2018; 538: 223-230.
- Aina M, Kuznyetsova D, Baillon F, Sescousse R, Sanchez-Ballester NM, Begu S, et al. Impact of disintegrants on rheological properties and printability in SSE 3D printing of immediate-release formulations. Eur J Pharm Sci. 2025; 206: 107017.
- Bin Zhang, Peter Belton, Xin Yi Teoh, Andrew Gleadall, Richard Bibb, Sheng Qi. An investigation into the effects of ink formulations of semi-solid extrusion 3D printing on the performance of printed solid dosage forms. J Mater Chem B. 2023; 12: 131-144.
- Tagami T, Ito E, Kida R, Hirose K, Noda T, Ozeki T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int J Pharm. 2021; 594: 120118.
- Conti S, Maggi L, Segale L, Ochoa Machiste E, Conte U, Grenier P, et al. Matrices containing NaCMC and HPMC 2. Swelling and release mechanism study. Int J Pharm. 2007; 333: 143-51.
- Sanubari Indah Putri Rahmani, Abdul Karim Zulkarnain. Optimization of HPMC and Na-CMC as Gelling Agents on Physical Properties and Stability in Sunflower Seed Oil Gel Formulation. J Food and Pharmaceutical Sci. 2023; 11: 812-819.
- Yang Y, Wang X, Lin X, Xie L, Ivone R, Shen J, et al. A tunable extruded 3D printing platform using thermo-sensitive pastes. Int J Pharm. 2020; 583: 119360.
- Cheng Y, Qin H, Acevedo NC, Jiang X, Shi X. 3D printing of extended- release tablets of theophylline using hydroxypropyl methylcellulose (HPMC) hydrogels. Int J Pharm. 2020; 591: 119983.
- Mengsuo Cui, Yining Yang, Danyang Jia, Pingfei Li, Qijun Li, Fen Chen, et al. Effect of novel internal structures on printability and drug release behavior of 3D printed tablets. J Drug Deliv Sci Technol. 2019; 49: 14-23.
- Hernández-Sosa A, Ramírez-Jiménez RA, Rojo L, Boulmedais F,Aguilar MR, Criado-Gonzalez M, et al. Optimization of the Rheological Properties of Self-Assembled Tripeptide/Alginate/Cellulose Hydrogels for 3D Printing. Polymers (Basel). 2022;1 4: 2229.
- Algahtani MS, Mohammed AA, Ahmad J, Saleh E. Development of a 3D Printed Coating Shell to Control the Drug Release of Encapsulated Immediate-Release Tablets. Polymers (Basel). 2020; 12: 1395.
- Dos Santos J, Balbinot GS, Buchner S, Collares FM, Windbergs M, Deon M, et al. 3D printed matrix solid forms: Can the drug solubility and dose customisation affect their controlled release behaviour? Int J Pharm X. 2022; 5: 100153.
- Farzana Khan Rony , Georgia Kimbell, Toby R. Serrano, Destinee Clay, Shamsuddin Ilias, Mohammad A. Azad. Extrusion-Based 3D Printing of Pharmaceuticals—Evaluating Polymer (Sodium Alginate, HPC, HPMC)-Based Ink’s Suitability by Investigating Rheology. Micromachines (Basel). 2025; 16: 163.
- Bin Zhang, Peter Belton, Xin Yi Teoh, Andrew Gleadall, Richard Bibb, Sheng Qi. An investigation into the effects of ink formulations of semi-solid extrusion 3D printing on the performance of printed solid dosage forms. J Mater Chem B. 2024; 12: 131-44.
- Prashant Polamaplly, Yiliang Cheng, Xiaolei Shi, Karthick Manikandan, Gül E. Kremer, Hantang Qin. 3D Printing and Characterization of Hydroxypropyl Methylcellulose and Methylcellulose for Biodegradable Support Structures. Procedia Manufacturing. 2019; 34: 552-559.
- Panraksa P, Udomsom S, Rachtanapun P, Chittasupho C, Ruksiriwanich W, Jantrawut P. Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer. Polymers (Basel). 2020; 12: 2666.
- Zidan A, Alayoubi A, Asfari S, Coburn J, Ghammraoui B, Aqueel S, et al. Development of mechanistic models to identify critical formulation and process variables of pastes for 3D printing of modified release tablets. Int J Pharm. 2019; 555: 109-123.
- Goole J, Amighi K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int J Pharm. 2016; 499: 376-394.
- Wang S, Chen X, Han X, Hong X, Li X, Zhang H, et al. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics. 2023; 15: 416.
- Solanki NG, Tahsin M, Shah AV, Serajuddin ATM. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J Pharm Sci. 2018; 107: 390-401.
- Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015; 217: 308-314.
- Abdella S, Afinjuomo F, Song Y, Upton R, Garg S. Mucoadhesive Buccal Film of Estradiol for Hormonal Replacement Therapy: Development and In-Vivo Performance Prediction. Pharmaceutics. 2022; 14: 542.
- Tugba Gulsun, Yagmur Akdag Cayli, Nihan Izat, Meltem Cetin, Levent Oner, Selma Sahin. Development and evaluation of terbutaline sulfate orally disintegrating tablets by direct compression and freeze drying methods. J Drug Delivery Science and Technology. 2018; 46: 251-258.
- González K, Larraza I, Berra G, Eceiza A, Gabilondo N. 3D printing of customized all-starch tablets with combined release kinetics. Int J Pharm. 2022; 622: 121872.
- Siow CR, Wan Sia Heng P, Chan LW. Application of freeze-drying in the development of oral drug delivery systems. Expert Opin Drug Deliv. 2016; 13: 1595-1608.
- Phang HC, Ng ZQ, Mohamad N, Chew YL, Balaraman A, Kee PE, et al. Comparison of oven drying and freeze drying methods for the production of fast melt films containing quetiapine fumarate. Drug Dev Ind Pharm. 2024; 50: 810-826.
- Rishi Thakkar, Amit Raviraj Pillai, Jiaxiang Zhang, Yu Zhang, Vineet Kulkarni, Mohammed Maniruzzaman. Novel on-demand 3-dimensional (3-d) printed tablets using fill density as an effective release-controlling tool. Polymers (Basel). 2020; 12: 1872.
- Bin Zhang, Jehad Nasereddin, Thomas McDonagh, Didier von Zeppelin, Andy Gleadall, FahadAlqahtani, et al. Effects of porosity on drug release kinetics of swellable and erodible porous pharmaceutical solid dosage forms fabricated by hot melt droplet deposition 3D printing. Int J Pharm. 2021; 604: 120626.
- References
- Elkasabgy NA, Mahmoud AA, Maged A. 3D printing: An appealing route for customized drug delivery systems. Int J Pharm. 2020; 588: 119732.
- Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M, et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). 2018; 6: 79-100.
- Su J, Yang L, Sun Z, Zhan X. Personalized Drug Therapy: Innovative Concept Guided With Proteoformics. Mol Cell Proteomics. 2024; 23: 100737.
- Eloise Neumann, Jason Patel. GUIDELINE FOR THE MANAGEMENT OF CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING. 2016.
- Antiemetic Guidelines for Chemotherapy and Radiation Therapy.
- Ashour AM. The preventive effects of ondansetron on chemotherapy- induced nausea and vomiting in adult cancer patients: systematic review from ClinicalTrials.gov. Front Pharmacol. 2023; 14: 1310455.
- Khan RB. Migraine-type headaches in children receiving chemotherapy and ondansetron. J Child Neurol. 2002; 17: 857-858.
- Christofaki M, Papaioannou A. Ondansetron: a review of pharmacokinetics and clinical experience in postoperative nausea and vomiting. Expert Opin Drug Metab Toxicol. 2014; 10: 437-444.
- Patel E, Rosemond D, Afzal A. Ondansetron induced torsades depointes. Clin Case Rep. 2019; 7: 1557-1558.
- Singh K, Jain A, Panchal I, Madan H, Gupta A, Sharma A. Ondansetron- induced QT prolongation among various age groups: a systematic review and meta-analysis. Egypt Heart J. 2023; 75: 56.
- Griddine A, Bush JS. Ondansetron. 2025.
- Chandrakala R, Vijayashankara CN, Kumar KK, Sarala N. Ondansetron induced fatal ventricular tachycardia. Indian J Pharmacol. 2008; 40: 186-187.
- Freedman SB, Uleryk E, Rumantir M, Finkelstein Y. Ondansetron and the risk of cardiac arrhythmias: a systematic review and postmarketing analysis. Ann Emerg Med. 2014; 64: 19-25.e6.
- Figg WD, Dukes GE, Pritchard JF, Hermann DJ, Lesesne HR, Carson SW, et al. Pharmacokinetics of ondansetron in patients with hepatic insufficiency. J Clin Pharmacol. 1996; 36: 206-215.
- Christian G. Daughton, Ilene Sue Ruhoy. Lower-dose prescribing: Minimizing “side effects” of pharmaceuticals on society and the environment. Science of The Total Environment. 2013; 443: 324-337.
- Allahham N, Fina F, Marcuta C, Kraschew L, Mohr W, Gaisford S, et al. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics. 2020; 12: 110.
- Ferreira Mariana, Lopes Carla Martins, Gonçalves Hugo, Pinto João F, Catita José. Personalised Esomeprazole and Ondansetron 3D Printing Formulations in Hospital Paediatric Environment: I-Pre-Formulation Studies. Applied Sciences. 2022; 12: 10585.
- Peng H, Han B, Tong T, Jin X, Peng Y, Guo M, et al. 3D printing processes in precise drug delivery for personalized medicine. Biofabrication. 2024; 16: 10.1088/1758-5090/ad3a14
- Funk NL, Fantaus S, Beck RCR. Immediate release 3D printed oral dosage forms: How different polymers have been explored to reach suitable drug release behaviour. Int J Pharm. 2022; 625: 122066.
- Ganatra P, Jyothish L, Mahankal V, Sawant T, Dandekar P, Jain R. Drug- loaded vegan gummies for personalized dosing of simethicone: A feasibility study of semi-solid extrusion-based 3D printing of pectin- based low-calorie drug gummies. Int J Pharm. 2024; 651: 123777.
- Ganatra P, Ashapogu A, Epili R, Dugam S, Desai J, Jain R, et al. Biomimetic replenishment therapy of cortisol using semi-solid extrusion - 3D printed tablets for adrenal insufficiencies. Int J Pharm. 2025; 672: 125342.
- Khaled SA, Alexander MR, Wildman RD, Wallace MJ, Sharpe S, Yoo J, et al. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int J Pharm. 2018; 538: 223-230.
- Aina M, Kuznyetsova D, Baillon F, Sescousse R, Sanchez-Ballester NM, Begu S, et al. Impact of disintegrants on rheological properties and printability in SSE 3D printing of immediate-release formulations. Eur J Pharm Sci. 2025; 206: 107017.
- Bin Zhang, Peter Belton, Xin Yi Teoh, Andrew Gleadall, Richard Bibb, Sheng Qi. An investigation into the effects of ink formulations of semi-solid extrusion 3D printing on the performance of printed solid dosage forms. J Mater Chem B. 2023; 12: 131-144.
- Tagami T, Ito E, Kida R, Hirose K, Noda T, Ozeki T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int J Pharm. 2021; 594: 120118.
- Conti S, Maggi L, Segale L, Ochoa Machiste E, Conte U, Grenier P, et al. Matrices containing NaCMC and HPMC 2. Swelling and release mechanism study. Int J Pharm. 2007; 333: 143-51.
- Sanubari Indah Putri Rahmani, Abdul Karim Zulkarnain. Optimization of HPMC and Na-CMC as Gelling Agents on Physical Properties and Stability in Sunflower Seed Oil Gel Formulation. J Food and Pharmaceutical Sci. 2023; 11: 812-819.
- Yang Y, Wang X, Lin X, Xie L, Ivone R, Shen J, et al. A tunable extruded 3D printing platform using thermo-sensitive pastes. Int J Pharm. 2020; 583: 119360.
- Cheng Y, Qin H, Acevedo NC, Jiang X, Shi X. 3D printing of extended- release tablets of theophylline using hydroxypropyl methylcellulose (HPMC) hydrogels. Int J Pharm. 2020; 591: 119983.
- Mengsuo Cui, Yining Yang, Danyang Jia, Pingfei Li, Qijun Li, Fen Chen, et al. Effect of novel internal structures on printability and drug release behavior of 3D printed tablets. J Drug Deliv Sci Technol. 2019; 49: 14-23.
- Hernández-Sosa A, Ramírez-Jiménez RA, Rojo L, Boulmedais F, Aguilar MR, Criado-Gonzalez M, et al. Optimization of the Rheological Properties of Self-Assembled Tripeptide/Alginate/Cellulose Hydrogels for 3D Printing. Polymers (Basel). 2022;1 4: 2229.
- Algahtani MS, Mohammed AA, Ahmad J, Saleh E. Development of a 3D Printed Coating Shell to Control the Drug Release of Encapsulated Immediate-Release Tablets. Polymers (Basel). 2020; 12: 1395.
- Dos Santos J, Balbinot GS, Buchner S, Collares FM, Windbergs M, DeonM, et al. 3D printed matrix solid forms: Can the drug solubility anddose customisation affect their controlled release behaviour? Int J Pharm X. 2022; 5: 100153.
- Farzana Khan Rony , Georgia Kimbell, Toby R. Serrano, Destinee Clay, Shamsuddin Ilias, Mohammad A. Azad. Extrusion-Based 3D Printing of Pharmaceuticals—Evaluating Polymer (Sodium Alginate, HPC, HPMC)-Based Ink’s Suitability by Investigating Rheology. Micromachines (Basel). 2025; 16: 163.
- Bin Zhang, Peter Belton, Xin Yi Teoh, Andrew Gleadall, Richard Bibb, Sheng Qi. An investigation into the effects of ink formulations of semi-solid extrusion 3D printing on the performance of printed solid dosage forms. J Mater Chem B. 2024; 12: 131-44.
- Prashant Polamaplly, Yiliang Cheng, Xiaolei Shi, Karthick Manikandan, Gül E. Kremer, Hantang Qin. 3D Printing and Characterization of Hydroxypropyl Methylcellulose and Methylcellulose for Biodegradable Support Structures. Procedia Manufacturing. 2019; 34: 552-559.
- Panraksa P, Udomsom S, Rachtanapun P, Chittasupho C, Ruksiriwanich W, Jantrawut P. Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer. Polymers (Basel). 2020; 12: 2666.
- Zidan A, Alayoubi A, Asfari S, Coburn J, Ghammraoui B, Aqueel S, et al. Development of mechanistic models to identify critical formulation and process variables of pastes for 3D printing of modified release tablets. Int J Pharm. 2019; 555: 109-123.
- Goole J, Amighi K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int J Pharm. 2016; 499: 376-394.
- Wang S, Chen X, Han X, Hong X, Li X, Zhang H, et al. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics. 2023; 15: 416.
- Solanki NG, Tahsin M, Shah AV, Serajuddin ATM. Formulation of 3DPrinted Tablet for Rapid Drug Release by Fused Deposition Modeling:Screening Polymers for Drug Release, Drug-Polymer Miscibility andPrintability. J Pharm Sci. 2018; 107: 390-401.
- Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015; 217: 308-314.
- Abdella S, Afinjuomo F, Song Y, Upton R, Garg S. Mucoadhesive Buccal Film of Estradiol for Hormonal Replacement Therapy: Development and In-Vivo Performance Prediction. Pharmaceutics. 2022; 14: 542.
- Tugba Gulsun, Yagmur Akdag Cayli, Nihan Izat, Meltem Cetin, Levent Oner, Selma Sahin. Development and evaluation of terbutaline sulfate orally disintegrating tablets by direct compression and freeze drying methods. J Drug Delivery Science and Technology. 2018; 46: 251-258.
- González K, Larraza I, Berra G, Eceiza A, Gabilondo N. 3D printing of customized all-starch tablets with combined release kinetics. Int J Pharm. 2022; 622: 121872.
- Siow CR, Wan Sia Heng P, Chan LW. Application of freeze-drying in the development of oral drug delivery systems. Expert Opin Drug Deliv. 2016; 13: 1595-1608.
- Phang HC, Ng ZQ, Mohamad N, Chew YL, Balaraman A, Kee PE, et al. Comparison of oven drying and freeze drying methods for the production of fast melt films containing quetiapine fumarate. Drug Dev Ind Pharm. 2024; 50: 810-826.
- Rishi Thakkar, Amit Raviraj Pillai, Jiaxiang Zhang, Yu Zhang, Vineet Kulkarni, Mohammed Maniruzzaman. Novel on-demand 3-dimensional (3-d) printed tablets using fill density as an effective release-controlling tool. Polymers (Basel). 2020; 12: 1872.
- Bin Zhang, Jehad Nasereddin, Thomas McDonagh, Didier von Zeppelin, Andy Gleadall, FahadAlqahtani, et al. Effects of porosity on drug release kinetics of swellable and erodible porous pharmaceutical solid dosage forms fabricated by hot melt droplet deposition 3D printing. Int J Pharm. 2021; 604: 120626.