Process Evaluation of a Randomized Controlled Trial Studying the Effect of Music Therapy in Patients with Huntington
- 1. Department of Neurology, Leiden University Medical Center, The Netherlands
- 2. ArtEZ University of the Arts, Department of Music Therapy, The Netherlands
- 3. Atlant Care Group, Apeldoorn, The Netherlands
- 4. KenVaK, Zuyd University, The Netherlands
- 5. Topaz Huntington Center Overduin, Katwijk, The Netherlands
- 6. Department of Public Health and Primary Care, Leiden University Medical Center, The Netherlands
Abstract
Background/Introduction: This article reports the process evaluation of a multi-center randomized controlled trial in which the effect of music therapy in Huntington’s Disease (HD) was studied. The beneficial affects of this complex intervention, recorded in many, mainly qualitative case reports and studies, could not be confirmed with the design and outcome measures used in the present study. To find possible explanations for this discrepancy, we performed a comprehensive process-evaluation.
Aim: To evaluate the execution of this Randomized Controlled Trial (RCT) by collecting information regarding (1) the study population, (2) the intervention and (3) the outcome measures. The evaluation will result in recommendations for future studies regarding study population, implementation and adjustment of the intervention and appropriate study design.
Method: A mixed model of qualitative and quantitative data was used. Qualitative data were analyzed following the guidelines of thematic analysis within the grounded theory and naturalistic inquiry. Quantitative data were derived from the evaluation forms and were accounted for in the analysis.
Results: The experience of twenty professionals was evaluated using an online survey, questionnaires, interviews, observation forms and field notes. The most obvious barrier was the severe cognitive decline of the study population in combination with circumstances within the participating centers, and the assessment tools that might not have been sensitive enough for the set goals of the RCT.
Conclusion: Performing a multi-center RCT studying the efficacy of music therapy with vulnerable patients in a long-term care facility is feasible, but challenging. No matter how complex the intervention and the study population, with the right study design, outcome measures and assessment tools, and with an unambiguous written protocol, and a continuous evaluation and monitoring throughout the whole study, barriers can be by-passed or avoided, and facilitators can be empowered. Suggestions are made for future research concerning the use of alternative designs (multiple single subject design study) and measurement tools (Goal Attainment Scale (GAS), Music Therapy Assessment Tool for Advanced HD (MATA HD).
Keywords
• Process evaluation
• Complex interventions
• Music therapy
• Huntington’s Disease
• Randomized controlled trial
Citation
Bruggen-Rufi van CHM, Hogenboom M, Vink AC, Achterberg WP, Roos RAC (2017) Process Evaluation of a Randomized Controlled Trial Studying the Effect of Music Therapy in Patients with Huntington’s Disease. J Mem Disord Rehabil 2(1): 1005
INTRODUCTION
This article reports the process evaluation of a randomized controlled trial (RCT) studying the effects of music therapy in patients with advanced stage Huntington’s disease. An extensive description of the protocol (methods, randomization procedure, and intervention) and the results of this RCT are published elsewhere [1,2]. Below is a short summary. Music therapy is considered a complex intervention. These are interventions comprising multiple components acting independently or interdependently, and therefore difficult to develop, document, evaluate and reproduce. It is increasingly recognized that especially these kinds of interventions should be rigorously evaluated [3]. Documenting and evaluating each process step in detail, exploring the execution, implementation, receipt and setting of an intervention will help the interpretation of outcome parameters and designing future trials [4]. Huntington’s disease (HD) is a progressive, neurodegenerative disease with autosomal dominant inheritance, characterized by motor disturbances, cognitive decline and behavioral and psychological symptoms and signs. These signs result in progressive functional decline [5] and a gradual loss of expressive and communicative skills. This combination leads to loss of quality of life [6]. Since there is no cure, all treatment is aimed at improving quality of life. One of these non-pharmaceutical treatments offered to patients with HD in long-term care facilities is music therapy. Music therapy is defined as “the clinical and evidence-based use of music interventions to accomplish individualized goals within a therapeutic relationship” [7]. Through music therapy an additional means of communication can be provided, enabling the patient to express his needs and emotions [8]. Furthermore, through music, contact can be established, especially as language deteriorates, such as for instance during the later stages of dementia [9]. The use and efficacy of music therapy in patients with Huntington’s disease on a large scale, has hardly been studied. Therefore, a multi-center single blind randomized controlled trial has been conducted between October 2014 and May 2016 in four long-term care facilities specialized in Huntington’s disease-care in The Netherlands [2]. The main goal of the RCT was to study the effect of music therapy on improving expressive and communication skills and behaviour in patients with advanced HD. Sixty-three patients were randomised using centre-stratified block-permuted randomisation. Two random groups were created. Over a period of 16 weeks, the experimental group was offered a weekly music therapy program according to a structured protocol, and the control group participated in weekly regular recreational therapy. Both therapies can be seen as complex intervention, difficult to develop, document, evaluate, and reproduce [10,11] (see box 1). In addition, both groups received usual care. The primary outcome measure to assess changes in expressive and communication skills was the second subscale (social cognitive functioning) of the Behavior observation Scale Huntington (BOSH) [12]. Changes in behavior were assessed by the third subscale (mental rigidity and aggression) of the BOSH and the Problem Behaviour Assessment-short version (PBA-s) [13]. The effect-evaluation showed that these outcomes did not improve in the MT group as compared to the control group [2]. This article reports the process evaluation of the aforementioned randomized controlled trial. The aim of the study is to evaluate the execution of this RCT by collecting information regarding (1) the study population, (2) the intervention and (3) the outcome measures. The outcome will result in recommendations for future studies in regard to study design, study population, implementation and adjustment of the intervention and outcome measures.
This study design as well as the music therapy intervention can be seen as an example of a complex intervention [10,11] because:
- It is a multi-center trial: the trial took place in four different long term care facilities.
- All four institutes had their own therapists, psychologists, physicians, and nursing staff who were involved in the trial. All professionals had to be instructed separately.
- Two different measurement tools had to be assessed by many different assessors at four different moments in a 28-week timeframe. All assessors had to be trained.
- The participants receiving the intervention all were diagnosed with Huntington’s disease in advanced stage, a disease with a wide spectrum of symptoms and signs.
- Interventions like music therapy are designed to be adapted to the specific needs of the patient (“tailor made”) and to local circumstances. Therefore, a high flexibility-rate was demanded from the music therapists.
METHOD
The method as well as the results and implications of the present process evaluation are presented using the format that is based on the following three components [10]
(1) The success rate of recruitment and quality of the study population;
(2) The quality of execution of the complex intervention, and;
(3) The process of acquisition of the evaluation data.
See for the related process measures of these three components box 2 [10].
Participants
Due to the vulnerability of the patients with HD in the advanced stage, we did not involve them in the process-evaluation: the burden of being exposed to additional questionnaires was an important consideration. Observation forms filled out by the therapists after each session were consulted to determine the patient’s compliance and experience. We used a purposive sampling strategy to select 20 professionals who had been involved in the execution of the trial. In doing this, we made sure that each group of professionals and each of the four participating centers were equally represented. Participating professionals were one team per site, consisting of a site monitor, a music therapist, a recreational therapist, a BOSH-assessor, and a PBA-s-assessor.
Data collection
A mixed method of qualitative and quantitative data collection was used to analyze the responses obtained from:
- Online survey of monitors, assessors and nursing staff and therapists;
- Evaluation forms filled out by music therapists and recreational therapists after each session;
- Interviews with one of each of the monitors, assessors, nursing staff and therapists;
- Notes taken during contact with monitors and research assistant
Online survey of monitors, assessors and nursing staff and therapists
An online survey was sent to all 20 participating professionals. Each of the three process components mentioned above was assessed in the questionnaire that consisted of ten open questions about the study population, the quality of execution of the complex intervention, and the process of acquisition of the evaluation data.
| Box 1: What makes an intervention complex [10,11] - Number of interacting components within the experimental and control interventions - Number and difficulty of behaviors required by those delivering or receiving the intervention - Number of groups or organizational levels targeted by the intervention - Number and variability of outcomes - Degree of flexibility or tailoring of the intervention permitted |
| Box 2: Process-evaluation components and related process measures of a complex intervention [10] Process Components Process Measures |
|
|
Study population
Multiple components
Evaluation data
|
1. Recruitment and selection rate
1. Quality of delivery of the interventional components
1. Outcome measures: coverage of interventional components |
Evaluation forms filled out by music therapists and recreational therapists after each session
Evaluation forms were filled out by the eight therapists involved in the study (four music therapists and four recreational therapists) after each session. These forms provided both qualitative and quantitative data about the treatment compliance of the patients, about disturbing factors prior to or during the intervention, and were used to check what activities and which techniques were used during the sessions and if this matched with the protocol.
Interviews with the monitors, assessors, nursing staff, and therapists
In addition to the questionnaires, we used face-to-face semi-structured interviews with four other professionals that were involved in the execution of the study: one BOSH-assessor, one PBA-assessor, one music therapist and one recreational therapist. They were chosen by way of convenience sampling. The three components mentioned above (study population, complex intervention and data-evaluation) were the guideline during the (semi-structured) interviews.
Notes taken during contact with monitors and research assistant
Furthermore, the four monitors of each participating center had contact with the principal researcher (MvBR) on a regular base throughout the whole study. This ensured the progress of the study, the adherence of the protocol and the data collection (i.e. random check of the raw scores in the data base). The frequency and the intensity of this contact differed between the sites. Also, the research assistant (MH) visited the four participating centers on a regular basis, checking the raw scores, and entering all collected data into SPSS version 22. Site notes of all her visits, phases of data collection and data preparation were also included in the present process evaluation.
Procedure Data collection
Guidelines of two methods were followed: the naturalistic inquiry [14] and the thematic analysis in accordance with the grounded theory of Glaser & Strauss [15]. The first is a method that emphasizes the trustworthiness of the study, while the second is an analytical approach used most frequently in grounded theory studies whereby theoretical insights are generated from qualitative data (inductive process) [16].
Trustworthiness
To ensure credibility and trustworthiness we used the following procedures:
Triangulation: We ensured methodological and data triangulation by using different methods of data collection (online survey, questionnaires, observation forms, interviews and site notes).
Confirmability: During the analysis, the research assistant (MH) examined the analysis process and records for accuracy (confirmability). The interpretations of the data and the preliminary results were presented and discussed with the principal researchers’ supervisors (AV, WA, RR) Their objective feedback was used to finalize the conclusions of the study.
Analysis
The analysis was conducted by the principal researcher (MvBR). The three components mentioned above [10] (study population, complex interventions and data-evaluation) were the guideline throughout the whole analysis procedure. The themes and concepts were derived from the data by using the open coding system: different categories were identified and codes that described the same themes were clustered or deducted (see figure 1a, 1b and 1c “Categories and Themes”).
Figure 1a: Categories and themes
Figure 1b: Categories and themes.
Figure 1c: Categories and Themes
A content analysis was performed according to the constant comparison method, based on the grounded theory of Glaser & Strauss [15]. Data were extensively collected, coded and organized. The whole process of constant comparison leads to general conclusion(s) which can logically be derived from the data [17,18].
RESULTS
Below, the results of the online survey (n = 19), the interviews (n = 4), the evaluation forms (n = 8) and the site notes (n = 5) are presented, using the format as depicted in box 2 [10]. See also Table (1)
Table 1. Demographic and clinical characteristics of the study population specified per institution.
| Inst. 1 MT (n = 9) | Inst. 1 RT (n = 9) | Inst. 2 MT (n = 4) | Inst.2 RT (n = 4) | Inst.3 MT (n = 11) | Inst.3 RT (n = 10) | Inst.4 MT (n = 8) | Inst.4 RT (n = 8) | Total MT (n = 32) | Total RT (n = 31) | |
| Number of sessions - attended/max - missed (%) |
90/144 54 (37,5%) | 102/144 42 (29%) |
58/64 6 (9,4%) |
55/64 9 (14%) |
166/176 10 (5,7%) |
146/160 14 (8,8%) |
96/128 32 (25%) |
80/128 48 (37,5%) |
410/512 102 (19,9%) |
383/496 113 (22,8%) |
| n (%) men n (%) women |
1 (11,1%) 8 (88,9%) | 1 (11,1%) 8 (88,9%) |
1 (25,0%) 3 (75,0%) |
1 (25,0%) 3 (75,0%) |
4 (36,4%) 7 (63,6%) |
4 (40,0%) 6 (60,0%) |
4 (50%) 4 (50%) |
4 (50%) 4 (50%) |
10 (50%) 22 (51,2%) |
10 (50%) 21 (48,8%) |
| Mean age (years) | 56 | 49 | 52 | 55 | 57 | 53 | 54 | 61 | 55 | 55 |
| Mean TFC-score at baseline |
0,22 |
1,88 | 0,75 | 1,75 | 1,09 | 2,33 | 1,88 | 1,5 | 0,99 | 1,86 |
| Mean MMSE-score - at baseline - after session 16 |
17,0 (n=3) 16,3 (n=3) | 20,4 (n=7) 20,1 (n=7) | 10,0 (n=3) 9,0 (n=3) | 20,0 (n=3) 22,2 (n=4) | 14,5 (n=9) 13,2 (n=9) | 16,7 (n=10) 14,4 (n=10) | 21,0 (n=2) 23,0 (n=2) | 20,0 (n=1) none | 15,6 (n=17) 15,4 (n=19) | 19,3 (n=26) 18,9 (n=21) |
| No. of patients (%) who received psychotropic medication throughout the duration of the trial (week 0 – week 28) |
7 (78%)
|
7 (78%)
|
2 (50%)
|
2 (50%)
|
11 (100%)
|
10 (100%)
|
5 (63%)
|
4 (50%)
|
25 (73%)
|
23 (70%)
|
| - antipsychotics - antidepressants - anxiolytics - hypnotics - anti-epileptics |
5 6 5 2 0 |
6 2 3 0 1 |
2 2 2 1 0 |
1 0 1 1 0 |
9 5 8 5 2 |
7 5 4 4 2 |
3 5 4 0 0 |
3 4 4 2 1 |
19 18 19 8 2 |
17 18 12 7 4 |
| Inst. = Institution, MT = Music Therapy, RT = Recreational Therapy | ||||||||||
(demographic and clinical characteristics of the study population specified per institution).
A. Study population
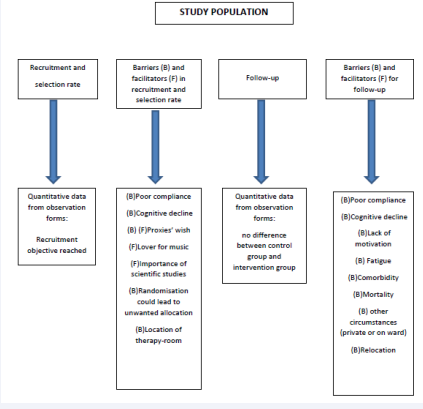
1. Recruitment and selection rate
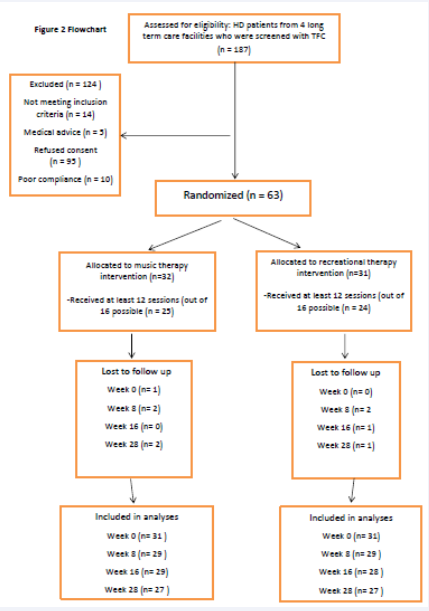
A total of 187 patients were eligible to participate in the trial see Figure (2)
Figure 2: Flow Chart
flowchart. 124 of them declined participation due to refusing consent (n= 95), not meeting the inclusion criteria (n=14), poor compliance (n=10) or medical advice (n=5). Most of the recruitment was done by the nursing staff. This process was complicated and time consuming because most of the study’s target population, patients in the advanced stage of HD, had poor insight and could not fully understand the purpose, the process and the possible risks and benefits of the study. In most cases, their proxies (next of kin or legal guardian) had to be informed as well. This happened both through information-meetings with the principal researcher, or by one-on-one meetings or telephone-calls by either the principal researcher or the nursing staff. Besides that, a written document that gave a comprehensive explanation about the study’s protocol was handed out to all eligible patients and their proxies.
Based on the statistical power calculation [1] we needed a minimum of 60 patients. 63 Patients or their proxies signed the informed consent: the recruitment objective was reached.
2. Barriers and facilitators in recruitment and selection process
The most important reasons for participation were the willingness to dedicate time and effort to scientific research. Also, the love for music was a great motivator to sign up. The most important reason why (the proxies of) eligible patients declined participation was the heavy burden to participate in scientific trials. Another hindrance was the chance to be allocated, through randomization, in the control group instead of the music therapy group: some patients would refuse to participate in the latter.
In the discussion section below we will elaborate on this topic.
Furthermore, in one of the participating centers, the room where the intervention was to take place was too small for patients in wheelchairs or beds, reason why some patients had to be excluded for participation beforehand.
3. Follow-up: attrition rate
Based on the quantitative data, the number of missed intervention-sessions in the experimental group (102/512) and in the control group (113/496) as well as missed assessments in the experimental group (19/256) and the control group (13/248) was practically equal. See also result C2.
4. Barriers and facilitators for follow-up
Reasons to miss sessions were relocation to another facility (mentioned 4 times), mortality (3), lack of motivation (2), fatigue (1), comorbidity (1) or private circumstances (1).
B. Multiple components of the complex intervention
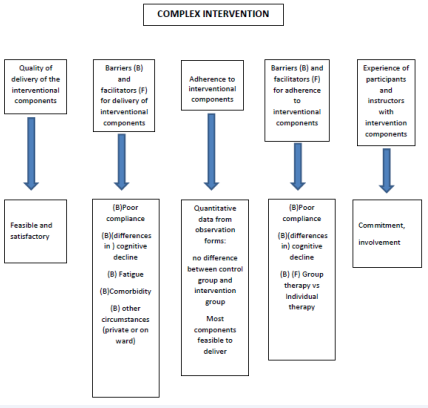
1. Quality of delivery of the interventional components
All eight therapists, who were extensively instructed before the start of the study and who followed the same protocol, stated that the feasibility and the satisfactory of delivering the intervention components were dependent on the differences in the cognitive state of the participating patients. For those patients, whose cognition was not severely affected, the sessions could be tailor made. For the others, however, delivering the planned components was not feasible in many occasions.
2. Barriers and facilitators for delivery of interventional components
The most important barrier for delivery and to fully benefit from the intervention, mentioned by all eight participating therapists, was the cognitive decline of the patients. Other barriers that were mentioned were equal to the reasons mentioned above why sessions were missed (see A4). An important barrier to deliver the intervention was the absence of the patients, due to the circumstances within the nursing homes: non-availability of the nursing staff to transport participants from the ward to the therapy room, broken elevators, or community-events (i.e. a concert or showing a movie) at the same time of the intervention. In one of the participating centers an extensive renovation was taking place at the same time as the study. This caused enormous noise pollution for both the experimental and control group. Based on the quantitative data derived from the observation forms, from the experimental group (MT), 22% of the participants followed fewer than 12 out of 16 sessions (n=7). For the control group (RT) this percentage was 23 see Table (1) and Figure (2) (n=7). An attendance of > 75% can be accounted for in the analyses of the outcome measures.
3. Adherence to interventional components
In both the experimental and control group, the interventional components were feasible to deliver to those patients who were actively involved. See B1.
4. Barriers and facilitators for adherence to interventional components
As mentioned above (see B2), the most obvious barrier for adherence was the low cognitive state some of the patients were in (mentioned by all eight therapists). Also, circumstances within the facility itself were important barriers for adherence.
5. Experience of participating therapists with intervention components
As mentioned above, the patients themselves did not receive questionnaires; hence they were not involved in the process-evaluation. According to the eight therapists who filled out the observation forms after each session, the patients with higher functioning cognition benefitted from the intervention, in that they enjoyed the sessions and that their communication and social interaction seemed to improve. All eight therapists felt a great commitment and involvement in the execution of the study. The protocol they had to follow was feasible, with enough opportunity to tailor it to meet the patient’s needs. As mentioned above, the biggest challenge for the therapists was the patients’ different cognitive state within the group. The overall opinion of the therapists was that the group size was small enough to warrant personal attention, despite this difference, while at the same time the group dynamic stimulated the social interaction of the patients.
C. Evaluation data
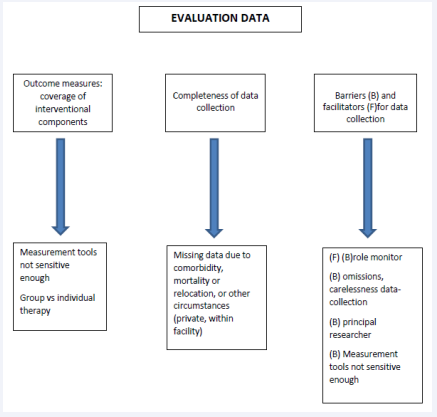
1. Outcome measures: coverage of interventional components
To make sure that all assessments took place at the four different time points, in the week prior to the assessment weeks (at baseline, week 8, 16 and 28), the monitors sent a reminder to all assessors. Afterwards, the monitors checked if all the measurements had taken place.
2. Completeness of data collection
The Behavior Observation Scale Huntington (BOSH) [12] was considered easy to administer by the nursing staff. No instruction was needed prior to the first assessment. However, if two answers were filled out instead of one when the assessors were not convinced about the answer, the least beneficial one was entered into the statistical program. This happened 56 times (0,8%). The assessors who administered the Problem Behaviour Assessment - short version (PBA-s) [13] had to follow a mandatory instruction-course, which they found very helpful. The frequency of both measurements (every 8 weeks, 3 times in total and a follow-up assessment after 12 weeks) was considered feasible. For the experimental group, 7 BOSH-assessments and 12 PBA-s-assessments were missed. For the control group these numbers were 8 for the BOSH and 5 for the PBA-s-assessments.
3. Barriers and facilitators for data collection
The frequency of both measurements (every 8 weeks, 3 times in total and a follow-up assessment after 12 weeks) was considered feasible but time-consuming for all assessors.
All four assessors that filled out the online survey stated that the PBA-s was more challenging to administer with the patients that were cognitively more declined. Besides that, for this tool a caregiver (nurse or proxy) had to be present to help answering the questions, which caused some more organization in planning. The assessors found the scoring of the PBA-s difficult, also due to the cognitive state of most of the patients. The mandatory training that they followed prior the study was considered very helpful.
Of the four participants who had administered the BOSH, one found the tool not sensitive enough for the more advanced patients. According to the assessors that filled out the survey, most important reasons why data were missing were participants moving to hospitals or other nursing homes or death.
Finally, the research-assistant, who was responsible for the data-input, encountered inconsistencies in the scoring or the collection of the data in two of the facilities. These omissions were not always picked up by the monitors who were responsible for the final check of the data before input into SPSS, and who otherwise could have instructed the assessors to be more accurate when filling out the forms.
DISCUSSION AND IMPLICATIONS
Barriers and facilitators of the study population
Results from the process evaluation indicate that some patients refused to participate once they were allocated to the control group and not to the music therapy group. The information about the trial has to be unambiguous that no changes between allocations can be made once the patient is randomized. Most studies that are similar to the present RCT use usual care or no treatment as control condition [19]. The present study used an active control condition providing similar amounts of attention and group contact for both groups. When conducting an RCT, the presence or absence of the treatment must be the only difference between the treatment and control groups in order to prove that any therapy works. While this is almost never the case when comparing music therapy to other therapies [20] recreational therapy was chosen as the control condition because being a complex, multi-faceted intervention as is music therapy, recreational therapy to us seemed to be the most appropriate control intervention to make the two groups as homogeneous in set-up and personal attention and thus as comparable as possible. The study-protocol unambiguously stated that no music activities were to be provided in the control group [2]. Apart from the intervention being compared, the groups were treated in an identical manner (i.e. day of the week, time of day, same “warm-up” and “cool-down” routine) and the same outcome data was obtained from all groups. Since the groups were randomly assigned and treated identically, apart from the intervention received, any differences in the outcomes are attributed to the difference in intervention [21,22]. However, randomization may not be appropriate If patients have a strong treatment preference. If they do participate, their motivation to remain in the study may drop significantly after learning that they have been assigned to the control group [21]. This was the case in the present study. Besides that, the cognitive problems, the lack of insight and the unawareness/anosognosia, known to patients with HD, often lead to poor treatment compliance [23,24]. Furthermore, there seemed to be a discrepancy between the proxies’ expectation and the patients’ ability or willingness to participate in the study. This implicates that more clearness has to be given, specifically to the proxies, about compliance to treatment end the burden to participate in the trial, and that inclusion of participants should specifically address appropriateness for participation, including physical and cognitive limitations [10].
Despite having executed a block-permuted randomization to allocate the participating patients, a difference in cognitive function in each group could not be avoided. This seemed to have been the most obvious limitation for the professionals to work with. Adjusting the baseline scores (TFC, MMSE) in the inclusion criteria could have avoided this difference. Classifying/dividing the groups on the basis of their baseline scores, however, will lead to non-homogeneous study-groups, and is therefore not desirable, nor is changing the cut-off score in the inclusion criteria, since the target group of the RCT are the more advanced HD-patients.
Delivery of the intervention
Process evaluation of the complex intervention in this study show that adherence and compliance leaves much to be desired, mostly due to comorbidity, mortality, relocation and circumstances on the ward where participants reside or the location of the therapy room in relation to the ward. The protocol should be clear about the location where the interventions are to take place; the treatment location should be close to the ward and accessible for all patients. A special consideration has to be given to caregivers’ availability to bring participants to and from the intervention room. Also, more emphasis should be placed on the importance of compliance, so that planning other events on the ward at the same time of the intervention will be avoided. The most important barrier for implementation of the intervention was the frailty of the participants. The signs and symptoms of patients with HD are mostly multifactorial by cause and include cognitive decline. Besides, many patients are receiving psychotropic medication see Table (1), resulting in a state of low awareness of their surroundings. The most important challenge for implementation of the intervention was the fact that the trial took place in four different facilities, each with its own therapist. Even though all therapists followed the same protocol, aiming for the same endpoints, the methods that were used varied in each house. The uniqueness of music therapy, however, i.e. the tailor- made interventions, allows this variety. The process-evaluation indicated that most therapists were satisfied with the delivery of their intervention, given the limitations they encountered as mentioned above.
Evaluation of the outcome measures
The process evaluation identified some limitations of the collection, evaluation and analyses of the outcome measures. Firstly, due to the fact that the patients were excluded for participation in the present evaluation, determining their reactions to the intervention is purely based on the perception of the therapists, hence subjective and possibly biased. The BOSH [12] is an observation form that is completely filled out by caretakers, while the PBA-s [13] has to be filled out with the help of patients and proxies. The numbers of missed measurements however were almost the same for the BOSH as for the PBA-s. There are indications that the measurement tools that were used might not have fully matched the intervention outcomes (improvement of communicative and expressive skills and behavioral changes). In future designs, adjusting the outcome measures to anticipated goals is desirable.
Strength and limitations
The strength of the presented process-evaluation is that the participating professionals, nor the principle researcher, had any knowledge about the results of the effect-study (the RCT): these results were deliberately withheld until initial data collection and analysis of this process-evaluation were complete, in order to prevent it from influencing the research findings. The process evaluation identified a valuable facilitator in the fact that each participating center had a monitor, a point of contact attached to the study. This was very beneficial for the process of collection and input of the data. The subjectivity of the interviewees and the persons surveyed can be seen both as a limitation and as strength of the present study. On the one hand they were to give answers concerning and on behalf of the participating patients, which could have led to bias. On the other hand, in qualitative research it is the subjectivity that matters the most, as social desirable responses are avoided.
Scoring the MMSE [25] at baseline and after the last session (week 16) was considered extremely difficult, due to the severe cognitive state the patients were in. Besides, the scoring was not consistent: some assessors (2) scored 0 where others (3) scored 99 (meaning the score are missing). These missing data were set to zero because to overcome this inconsistency in the scores between the houses. This may have led to a distorted image of the MMSE-outcomes. Instructions hereabout must have been better described in the protocol. Finally, all participating professionals stated that they felt involved in and committed to both the process evaluation and the effect-study. At the same time, however, the contact with the principal researcher was sometimes laborious, due to the fact that the latter was not often present/visible on site. Most of the contact had to be maintained through internet.
CONCLUSION AND RECOMMENDATIONS FOR FUTURE STUDIES
Based on the results of the process-evaluation, the limitations in the present study are:
- The cognitive state of the patients: the highly fluctuating physical and emotional responses to the intervention, and the diverse demographical, psychosocial and musical backgrounds of the patients, mean that randomization is likely to be ineffective in distributing confounders evenly between groups.
- The use of quality of life outcome did not necessarily capture respondents’ characteristic voices reflecting whether the experience was important to them. Furthermore, the treatment goal was the same for all participating patients: improving communicative and expressive skills.
- The music therapy protocol could not be standardized, as music therapists tailor their approach according to patient’s need.
For future study designs, the following possibilities can be taken into consideration to avoid these barriers and limitations:
- Individual therapy sessions: the power of music therapy lies in the fact that the sessions can be tailor-made, meeting the individual needs of each patient [1,2]. Taking the differences in cognitive state of the group-members into consideration, individual therapy to deliver the planned intervention components is recommended.
- Using the Goal Attainment Scale (GAS) as the primary assessment tool: Goal attainment scaling is a mathematical technique for quantifying the achievement of personalized goals that are set for each individual that is included in the study [26].
- Using the Music Therapy Assessment Tool for Advanced Huntington’s Disease (MATA-HD) as the secondary assessment tool. A pilot validation study of this newly developed tool has just finished in the UK [27]: preliminary data indicate that the MATA-HD is a promising tool for measuring patient responses to music therapy interventions across psychological, physical, social and communication domains of functioning in patients with advanced HD. The MATA-HD was not available yet when designing and executing the present study.
- Furthermore, validated measurement tools that are sensitive for emotional and social cognitive responses in dementia might be suitable to use in future studies with HD-patients.
- Designing a multiple single subject design study: this is a research design often used in applied fields of psychology and human behavior in which the subject serves as his/her own control, rather than using another individual or group. These designs are highly flexible and highlight individual differences in response to intervention effects [28].
The demands for evidence of treatment efficacy and effectiveness are placing increased pressure on the field of music therapy. Although the dialogue of clinical effectiveness in music therapy should not be dominated by the biomedical hierarchical model of evidence-based practice, “despite the challenges of meeting all key design demands typical of an RCT, it is possible to design rigorous music therapy RCTs that accurately estimate music therapy treatment benefits” (Bradt, 2012, p. 120) [21]. Performing a multi-center RCT studying the efficacy of music therapy with vulnerable patients in a long-term care facility is feasible but challenging, if certain conditions are met and adjustments made. No matter how complex the intervention and the study population, with an unambiguous written protocol, the right study design, outcome measures and assessment tools, and a continuous evaluation and monitoring throughout the whole study, barriers can be by-passed or avoided, and facilitators can be empowered. When evaluating interventions that have the potential to improve quality of life, finding the best research designs and the best outcome measures for patients in the advanced stage of HD remains a major challenge. The biggest challenge for the music therapy researcher is to integrate different study-designs and to learn from the experiences of previous complex intervention studies. This asks for all multidisciplinary team members surrounding the patient with Huntington’s disease to be willing to learn, to cooperate and to be creative [29].
AUTHORS’ CONTRIBUTIONS
MB is a neurological music therapist fellow. She developed the study design and drafted the manuscript.
MH is a psychologist. She contributed to drafting (part of) the manuscript.
AV is a psychologist. She reviewed the manuscript.
WA is elderly care physician and professor of institutional and elderly care medicine. He contributed to the development of the study design and reviewed the manuscript.
RR is a neurologist and contributed to the development of the study design and reviewed the manuscript.
All authors have been involved in reviewing the manuscript, and have read and approved the final text.
FUNDING
This study was supported in part by a grant of the Jacques and Gloria Gossweiler Foundation (JGGF), Switzerland. The funding source had no role in the study design nor in the execution, analyses, interpretation of the data, writing the manuscript or the decision to submit the manuscript for publication.