The Role of Extended Release Guanfacine in the Management of ADHD
- 1. Department of Clinical and Experimental Medicine, University of Catania, Italy
Abstract
Guanfacine extended release (GXR) is a a2A-adrenoreceptor agonist approved by the European Medicines Agency (EMA) in September 2015 for treatment of attention-deficit/hyperactivity disorder (ADHD) in child and young people (CYP) aged 6–17 year, when stimulants are not effective or are not a good choice for patients and/ or families. The EMA suggests that GXR should be a part of a multimodal approach to ADHD, with psycho educational support. The present article reviews the chemical, pharmacodynamics, and pharmacokinetics properties of GXR and discussed the results of randomized controlled trials on treatment of ADHD in CYP. The scientific literature supports GXR as good option for ADHD in youth for its safety and efficacy.
Citation
Rizzo R, Gulisano M, Ferro MC, Domini CN (2017) The Role of Extended Release Guanfacine in the Management of ADHD. J Mem Disord Rehabil 2(1): 1003.
Keywords
• Guanfacine extended release
• Attention deficit hyperactivity disorder
• a2A adrenoceptor agonist
• pharmacological treatment
ABBREVIATIONS
GXR: Guanfacine Extended Release; EMA: European Medicines Agency; ADHD: Attention-Deficit/Hyperactivity Disorder; CYP: Child and Young People; PD: Personality Disorders; CD: Conduct Disorders; OCD: Obsessive Compulsive Disorder; RCTs: Randomized Controlled Trials; ER: Extended-Release; ADHD-RS-IV: ADHD Rating Scale-IV; CGI-I: Clinical Global Impression of Improvement; PGA: Parent’s Global Assessment; CPRS-R:SF: Conners’ Parent Rating Scale-Revised: Short Form; CTRS-R:SF:Conners’ Teacher Rating Scale-Revised: Short Form; CRT: Choice Reaction Time; WFIRS-PR: Weiss Functional Impairment Rating Scale-Parent Report; PTF: Percentage of Treatment Failure; TIF: Time of Treatment Failure; AEs: Adverse Events
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is a complex neurobehavioral disorder characterized by pervasive and persistent developmentally inappropriate inattentiveness, impulsivity, and hyperactivity. According to DSM-5 criteria, symptoms must persist for at least 6 months, interfere with functioning in at least 2 settings (eg social, academic, or occupational), and not caused by another psychiatric disorder [1]. 3 subtypes have been described: hyperactive/impulsive, inattentive, and combined [1].
ADHD is a common childhood neuro developmental disorders; its worldwide prevalence is between of 5–8% in CYP and 2.5–4% in adults [2-4]. Additionally, ADHD is frequently associated with co morbid psychiatric disorders: 33% of children have 1 comorbid disorder, 16% have 2, and 18% have 3 or more. Patients with ADHD have higher rates of, problems at school and failures, social problems and impaired quality of life [5]. Furthermore, 50% of CYP with ADHD also presented tic disorders [6].
It has recently been reported that 21.6% of patients with ADHD have comorbid personality disorders (PD), 17.6% have conduct disorders (CD), and 4% have both PD and CD [7]. Additionally, 46% of patients with ADHD have a learning disability and 12% have speech problems, [8] 27% present with anxiety, 18% have depression, and 15% have both anxiety and depression [9]. Co morbidity with obsessive compulsive disorder (OCD) is highly inconsistent, ranging from 0–60%; a lower incidence was reported in adults compared to CYP [10].
ADHD has multifactorial origins, with contributions from biological, genetic, and environmental factors [4]. It is generally accepted that imbalances in dopaminergic, noradrenergic, and other neurotransmitter systems in the brain contribute to the behavioral sequelae that characterize ADHD. Therefore, the mechanisms of action for medications that effectively treat ADHD are hypothesized to increase levels of neurotransmitters (specifically nor epinephrine and/or dopamine, or their precursors) at the synapse either by facilitating release, decreasing reuptake, or by binding and activating post-synaptic receptors [11].
Currently, there are both non-pharmacological and pharmacological treatment options (including stimulants and non-stimulants) for ADHD [12]. Psycho stimulant medications are the most common drug prescribed in the treatment of ADHD because their large affect sizes and short effect latencies [13]. Nevertheless in approximately 25–30 % of CYP with ADHD, stimulants are not effective, or parents/caregivers may prefer a non-stimulant medication. Huss et al. suggested that extended-release guanfacine (CO2ACO2; GXR) could be a good alternative therapy for patients with poor response to stimulants, Tourette disorder, emotional dysregulation, oppositional defiant disorder, intolerable side effects from stimulants, hypertension/other cardiovascular disease, substance use disorder, and circadian cycle/sleep problems [14-15]. In adulthood, untreated ADHD, is recognized as an important risk factor for several psychiatric conditions, and is associated with behavioral problems; conduct disorders and poor quality of life [16]. Therefore, physicians and parents/caregivers should consider the benefits and risks associated with treating ADHD symptoms, compared to those of non-treatment [17,18].
The present review focuses on the extended-release formulation of guanfacine (GXR), authorized by the European Medicines Agency (EMA) in September 2015 for treatment of ADHD in CYP aged 6–17 years [19]. The EMA suggests that GXR should be a part of a multimodal approach to ADHD, with psycho educational support when stimulants are not effective or are not a good choice for patients and/or families [19].
The present study reviews the chemical, pharmacodynamics, and pharmacokinetics properties of GXR and discussed the results of randomized controlled trials (RCTs) on treatment of ADHD in CYP. The literature search included studies published prior to February 2017, and was conducted on MEDLINE and EMBASE.
Chemical properties
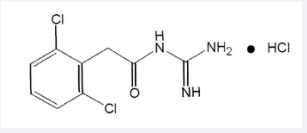
GXR is a once-daily, extended-release (ER) matrix tablet formulation of guanfacine; it is N-amidino-2-(2,6-dichlorophenyl) acetamide mono hydrochloride, its molecular formula is C9 H9 Cl2N3 O • HCl, with a molecular weight of 282.55. Chemical structure of GXR is shown in (Figure 1)
Figure 1: Chemical structure of guanfacine extended release.
Pharmacodynamic properties
Guanfacine is a selective α2A-adrenergic receptor agonist; it has been studied as antihypertensive with central action (ATC code, C02AC02). Additionally, guanfacine is a non-stimulant, used for the treatment of ADHD even if its mechanism of action has not been fully established. Preclinical studies have supposed that guanfacine modulates neither signaling in the prefrontal cortex and basal ganglia through direct modification of synaptic nor epinephrine transmission at α2-adrenergic receptors [20].
Pharmacokinetic properties
GXR is administered once daily, its formulation allows gastrointestinal extended release [21]. GXR is available in tablets (1, 2, 3, 4 mg). The recommended daily dose is between 1 and 4 mg; no data about safety and efficacy are reported for doses >4 mg/day. Furthermore, the suggested dose increase is 1 mg/week, when lower doses are well-tolerated [22].GXR is easily absorbed; in CYP peak plasma concentrations, after oral administration, is reached in about 5 hours. Approximately 70% of guanfacine is bound to plasma proteins and metabolized via CYP3A4/5- mediated oxidation, with subsequent reactions of sulfation and glucuronidation and elimination half-life of approximately 18 hours. The major circulating metabolite is 3-OH-guanfacine sulphate [22]. 80% of guanfacine excretion is by kidneys (filtration and active secretion), and the remaining 20% by the liver. The major urinary metabolites are 3-hydroxy guanfacine glucuronide, guanfacine dihydrodiol, and 3-hydroxy guanfacine sulfate [22]. No pharmacokinetics differences were reported in children (6–12 years of age) and adolescents (13–17 years of age) affected by ADHD, as well as in healthy adult volunteers. No differences were reported between systemic exposure in men and women, with the same mg/kg dose. No studies reported about GXR pharmacokinetic in children <6 years of age [19].
Efficacy
The effects of GXR were assessed in CYP with ADHD, through several RCTs sponsored by industries. Biederman et al., conducted an 8 weeks multicenter, double-blind, placebo-controlled, fixed-dosage escalation study in patients aged 6–17 years with ADHD [23].
345 patients were recruited and randomly assigned to 2, 3, or 4 mg/day of GXR, or placebo. The efficacy was evaluated using the ADHD Rating Scale-IV (ADHD-RS-IV) as primary measure and the Clinical Global Impression of Improvement (CGI-I), Parent’s Global Assessment (PGA), Conners’ Parent Rating Scale-Revised: Short Form (CPRS-R: SF), and Conners’ Teacher Rating Scale-Revised: Short Form (CTRS-R: SF) as secondary measurements. Statistically significant differences were found in ADHD-RS-IV score changes from baseline to the endpoint (on the hyperactivity/impulsivity and inattentiveness subscales), between GXR groups and control group. Moreover GXR groups showed significant improvements of the CGI-I, PGA, CPRS-R: SF, and CTRS-R: SF, compared to the placebo group. GXR was effective, as demonstrated from both primary and secondary measurements, compared to placebo, and well tolerated [23].
Sallee et al., conducted a 9 weeks, multicenter (52 sites) randomized, double blind placebo controlled study in the United States. 322 patients with a DSM IV-TR diagnoses of ADHD were recruited and randomly assigned to GXR 1, 2, 3, or 4 mg/day or placebo [24].
The efficacy was evaluated using the ADHD Rating Scale-IV as primary measure and with the scores on the hyperactive/ impulsive and inattentive subscales of the ADHD-RS-IV, clinician and parent ratings, duration of clinical effect, and safety as secondary measurements. Statistically significant differences were found between GXR and placebo groups with a reduction in ADHD-RS-IV score changes from baseline to the endpoint [24].
Connor et al., conducted a 9-week, multicenter (33 sites) flexible dose-optimization RCT study, in the USA, to evaluate GXR for treatment of oppositional symptoms in 6–12-year-old children with ADHD [25]. 217 children were enrolled, and randomly assigned to the GXR (n = 138) or placebo (n = 79) group. The efficacy was evaluated using the oppositional subscale of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R: L) score as primary measure and ADHD-RS-IV scores as secondary measure. A statistically significant reduction from the baseline to the end point was found on the CPRS-R: L oppositional sub scale, and ADHD-RS-IV scores in patients assigned to GXR group compared to placebo.
A post hoc correlation analysis between percentage reduction from baseline to the study conclusion on the CPRS-R: L oppositional subscale and total scores on the ADHD-RS-IV indicated that the decreases in oppositional symptoms and ADHD symptoms were highly correlated (r = 0.74). Therefore, the findings support efficacy of GXR in treating children with ADHD and oppositional symptoms [25].
Kollins et al., conducted a 6-week multicenter (9 sites) study in the USA. This RCT included 182 CYP affected by ADHD [26]. The authors want to evaluate whether effective doses of GXR in patients with ADHD could cause impairment in cognitive tasks, alertness, or affected daytime sleepiness. The measures assessed were: the Choice Reaction Time (CRT) test from and the Cambridge Neuropsychological Test Automated Battery for psychomotor functioning and alertness. Standard efficacy measures related to ADHD were also included. No statistically significant differences were reported between GXR and control group with regards to psychomotor functioning or alertness and daytime sleepiness from the beginning to the conclusion of the study. The authors concluded that, at effective doses for ADHD symptoms, GXR did not differ from placebo with regard to cognitive impairment, alertness and daytime sleepiness and for these reason sedation was not related to GXR administration [26].
Sallee et al., conducted a post-hoc analysis on the efficacy of GXR [27] comparing data from 2 large previous double-blind placebo-controlled trials, using ADHD-RS-IV score [23,24]. They studied 631 patients between 6 and 17 years; 490 received GXR and 141 received placebo. Moreover participants were divided in 3 ADHD subgroups: “predominantly inattentive” (127 patients), “combined” (354 patients) and “predominantly hyperactive-impulsive” (9). After a pooled analysis AA found that patients treated with GXR affected by “predominantly inattentive” and “combined” ADHD subtypes showed statistical significant lower ADHD-RS-IV score compared to placebo control group. With regards to patients with “predominantly hyperactive-impulsive” ADHD subtypes no results were reported because of the small sample size [27].
Newcorn et al., conducted an 8 week, multicenter (47 sites) randomized, dose-optimization, double blind placebo controlled study in the United States and Canada. 333 children between 6 and 12 years of age, affected by ADHD defined by DSM-IV-TR criteria were recruited and randomly assigned to GXR (1–4 mg/day) upon awakening in the morning plus a placebo in the evening (107 participants), placebo in the morning plus GXR in the evening (114 participants), or twice-daily placebo (112 participants).
After treatment AA found that GXR mono therapy (both in the morning and in the evening administration) was related to statistical significant decrease of the ADHD-RS-IV scores, compared to placebo with a good effect size [28].
Hervas et al., conducted a multicenter (Europe, USA and Canada), randomize controlled phase III study to assess efficacy (symptoms and function) and safety of dose-optimized GXR compared to placebo; a reference arm of atomoxetine compared with placebo was used [29].
338 CYP aged 6-17 years were recruited and randomly assigned to either dose-optimized GXR (0.05–0.12 mg/kg/day; ages 6–12 years, 1–4 mg/day; ages 13–17 years, 1–7 mg/day), ATX (10–100 mg/day), or placebo, for 4 or 7 weeks.
The efficacy was measured using the ADHD-RS-IV score as primary measure and the CGI-I and the Weiss Functional Impairment Rating Scale-Parent Report (WFIRS-PR) (learning and school, and family domains) as secondary measurements. Significant differences were observed form the baseline to the endpoints in all measures.
AA concluded that GXR is effective treatment for severe- mild ADHD core symptoms and global patient functions [29].
Young et al., conducted a multicenter, double-blind, placebo-controlled dose optimization, phase III study to evaluate the efficacy of once-daily GXR monotherapy administered either in the morning or evening in children with ADHD. The efficacy was evaluated using the ADHD-RS-IV as primary measure (previously examined by Newcorn et al., 2013 [27]. and the CPRS-R: S, (which was administered 3 times/day in the morning, afternoon, and evening prior to each study visit) as secondary measure [30].
333 Children aged 6–12-years with ADHD were recruited and randomized into 3 groups: GXR in the morning and placebo in the evening (n = 107), placebo in the morning and GXR in the evening (n = 114), or twice-daily placebo (n = 112). At the end of the study children receiving GXR had statistically significant improvement from baseline in daily mean total CPRS-R:S scores, as well as in each of the morning, afternoon, and evening CPRS-R:S assessments, compared to participants in the placebo group [31].
Stein et al., conducted an 8-week, multicenter (47 sites), double-blind, placebo-controlled, dose-optimization study in USA and Canada, in children aged 6–12 years with ADHD based on ADHD-RS-IV scores > 28 and CGI-I scores ≥ 4 [31]. 333 children were recruited and randomly assigned (1:1:1) into 3 groups: GXR 1–4 mg/day in the morning plus placebo in the evening (111), GXR 1–4 mg/day in the evening plus placebo in the morning (111), or twice-daily placebo (111). The efficacy was measured with the ADHD-RS-IV as primary measure and with the WFIRS-P as secondary measure. AA focused their attention on secondary measure and they found that children treated with GXR (both in the morning and in the evening) presented statistically significant reductions in WFIRS-P functional impairment scores compared to with placebo treatment. Moreover they observed that changes in WFIRS-P scores were congruent with clinical responses, as determined by both ADHD symptom reduction (measured with ADHD-RS-IV) and CGI-I scores [31]
Wilens et al., conducted a 13-week phase 3 multicenter, randomized, double-blind, placebo-controlled trial evaluating the efficacy of once/daily GXR (1–7 mg/day) in adolescents aged 13–17 years with the diagnosis of ADHD [32].
314 patients were recruited and randomly assigned into 2 groups GXR (157) and placebo (157). The efficacy was measured using the ADHD-RS-IV as primary measure and the CGI-I as well as the learning and school domain and family domain on the WFIRS-P as secondary measurements. After GXR treatment adolescents showed statistically significant improvement in total ADHD-RS-IV scores, GCI and WFIRS-P subscales compared to those in the placebo group [32].
Newcorn et al., conducted a phase 3, double-blind, placebo-controlled, randomized-withdrawal study evaluating the long-term efficacy of GXR in CYP with ADHD [33].
528 CYP aged 6–17 years were recruited and received open-label GXR (1–7 mg/day). After 13 weeks, 316 responders (59.8%) were randomized to GXR or placebo in the 26-week, double-blind, randomized-withdrawal phase (RWP). The efficacy was measured with the percentage of treatment failure (PTF), as primary measure, and the time of treatment failure (TTF), as secondary measure. AA found that the PTF was statistically significant higher in placebo group compared to GXR group. With regards to the TTF it was statistically significant longer in CYP treated with GXR compared to placebo control group [33].
Huss et al., conducted a RCT to assess the efficacy of GXR relative to prior stimulant treatment, in which ATX was included as a reference for efficacy, in an open-label phase of a RWS [34]. AA concluded that GXR response was not related to prior stimulant treatment; however, ATX produced improvement only in stimulant-naïve participants relative to placebo. These results may be relevant to clinical decisions regarding ADHD treatment sequencing [34].
Recent research had, also, evaluated the efficacy of guanfacine as an adjunctive pharmacotherapy in patients treated with stimulants [24]. For patients with inadequate responses to stimulants, combining stimulants and adjunctive medication may improve ADHD symptom management, reduce dose-limiting adverse events, and reduce co morbidities [35]. These results are very important, and suggest that guanfacine may improve ADHD symptoms in patients treated with stimulants [36,37].
Finally, GXR has been proposed as an adjunctive treatment to improve stimulant adherence in children with ADHD. Preliminary data suggest a positive effect of GXR, although more research is needed (unpublished data).
Safety and tolerability
The most common adverse events (AEs) reported in the literature were: sedation, somnolence, fatigue, drowsiness, headache, and upper abdominal pain [37]. The EMA product report section for GXR indicates that very commonly reported AEs observed in controlled, double-blind, open-label clinical studies include: somnolence (40.6%), headache (27.4%), fatigue (18.1%), upper abdominal pain (12.0%), and sedation (10.2%). Serious AEs including hypotension (3.2%), weight increase (2.9%), bradycardia (1.5%), and syncope (0.7%) were reported and classified as uncommon [19].
In a recent review, Martinez-Raga et al., reported that the most common AEs in short-term RCTs of GXR compared to placebo were somnolence (27–50.7% vs. 3.5–22.8%), headache (16.7–26.3% vs. 10.6–24.3%), upper abdominal pain (6.1–14.3% vs. 2.6–9.1%), fatigue (10.9–25.4% vs. 2–18%), and sedation (5.9–14.5% vs. 2.7–4.5%) [4]. Similar rates were reported for long-term RCTs [4].
Sedation is generally mild to moderate in severity and occurs within 2–3 weeks of starting treatment. It is also typically dose-related and decreases over time. Martin et al suggested that the antihypertensive effects of GXR are associated with dose-dependent decreases in blood pressure and heart rate in adolescents with ADHD, which is confirmed by the majority of RCTs [33].
Additionally, bradycardia and QTc prolongation were observed in the majority of RCTs [30-32]; however, none of the observed prolongations were clinically significant. Prolonged bradycardia and hypotension were reported in a case of GXR overdose [37], however, significant QTc increases did not occur in other studies [27,32,36]. Potential prolongations should be considered in children who are known to have prolonged QTc intervals.
Table 1: Randomized controlled trials on extended release guanfacine (GXR).
| Author (year) | N | Mean age/ range (years) | Drug dose | Study duration (weeks) | Adverse events |
|
Biederman et al (2008)
|
345
|
10.5/6–17
|
2 mg
|
8
|
Headache, somnolence, fatigue, upper abdominal pain, and sedation. Small to modest changes in blood pressure, pulse rate, and electrocardiogram parameters that were not clinically meaningful. |
|
Sallee et al (2009)
|
324
|
11.0/6–17
|
1 mg 2 mg 3 mg 4 mg placebo |
9
|
Somnolence, headache, fatigue, sedation, dizziness, irritability, upper abdominal pain, and nausea. Somnolence, sedation, and fatigue within the first 2 weeks. |
|
Connor et al (2010)
|
217
|
9.4/6–12
|
1–4 mg/day (mean 2.87 mg/day) placebo |
9
|
Somnolence, headache, sedation, upper abdominal pain, and fatigue (mild/moderate), which were typically during dose-titration. Modest changes in blood pressure, pulse rate, and ECG that were not clinically significant. |
|
Kollins et al (2011)
|
182
|
12.6/6–17
|
1–3 mg/day (mean 2.46 mg/day) placebo |
6
|
Sedative adverse events (mild/moderate) during dose titration. One participant in the GXR group discontinued due to fatigue and somnolence. |
|
Sallee et al (2012)
|
631
|
10.5; 11 6–17
|
1 mg
|
8/9
|
Somnolence, headache, fatigue, sedation, dizziness, irritability, upper abdominal pain, and nausea. Somnolence, sedation, and fatigue within the first 2 weeks. Small to modest changes in blood pressure, pulse rate, and electrocardiogram parameters that were not clinically meaningful. |
| Newcorn et al (2013) |
333
|
9.1/6–17 |
AM 1–4 mg/ day PM 1–4 mg/ day |
8
|
Somnolence (mild/moderate). |
|
Hervas et al (2014)
|
338
|
10.2/6–17
|
ATX = 112 (10–100/mg day) GXR = 114 1, 2, 3, 4 mg/ day) placebo |
10 (6–12 years) 13 (13–17 years) |
Somnolence, headache, fatigue (mild/moderate), and syncope. Modest changes in blood pressure, pulse rate, and ECG that were not clinically significant. |
|
Young et al (2014) & Stein et al (2015)
|
333
|
9.1 GXR AM 9.3 GXR AM 8.9 PLA 6–12 |
GXR AM = 107 GXR PM = 114 placebo = 112 GXR: 3–4 mg/ day |
8
|
Somnolence (mild/moderate). |
|
Wilens et al (2015)
|
314
|
13–17
|
GXR 1–7 mg/ day = 157 placebo = 157 |
13
|
Most treatment-emergent adverse events were mild to moderate, with sedation-related events most commonly reported. |
|
Newcorn et al (2016)
|
528
|
10.2
|
GXR 1, 2, 3, 4 mg/day placebo
|
13 OL
|
Severe adverse events in 5 participants: syncope, sinus bradycardia, and somnolence. Headache, sedation, upper abdominal pain, and fatigue (mild/moderate). |
|
Huss et al (2016)
|
337
|
6–17
|
GXR 1–7 mg/ day ATX 10–100 mg/day placebo |
13
|
Severe adverse events in 5 participants, which resulted in discontinuation by 3 participants. |
| Abbreviations: ATX = Atomoxetine; GXR= Guanfacine Extended Release | |||||
CONCLUSION
GXR is an effective and safe non-stimulant treatment option for ADHD symptoms in CYP, as mono therapy or as adjunctive therapy with psycho stimulants. GXR has the advantage of increased patient compliance because it’s once-daily dosing. Both the literature and recommendations by the EMA indicate that treatment must occur under the supervision of an expert child and adolescent neuropsychiatry. Pre-treatment screening is mandatory; a baseline evaluation to identify patients at risk of somnolence and sedation, hypotension and bradycardia, QT-prolongation arrhythmia, or weight gain /risk of obesity is recommended. A detailed family history in order to identify cardiac disease, sudden deaths or growing deficits is recommended too. Careful dose titration and monitoring is necessary at the beginning of GXR treatment, since clinical improvement and risks for several clinically significant adverse reactions (syncope, hypotension, bradycardia, somnolence, and sedation) are related to both dose and the duration of exposure. Patients should be advised that somnolence and sedation may occur, particularly early in treatment or with dose increases. If somnolence and sedation are clinically concerning or persistent, a dose decrease or discontinuation should be considered. We acknowledge some limitations of our review. The main limitation is that we have restricted our discussion to data on GXR use in children and adolescents. Additionally, we reviewed studies that had relatively short durations of observation. The EMA acknowledges that GXR use must be further evaluated in order to elucidate the long-term efficacy, as well as the effects as they relate to the course of symptoms.
DISCLOSURE
The authors did not receive any financial support or writing assistance in the preparation of this manuscript. AA report no financial interests or potential conflicts of interest. Dr. R. Rizzo and Dr. M. Gulisano were investigators on guanfacine trial SPD503-318.