Vaccination Coverage in Children with Rheumatic Diseases at a Tertiary Pediatric Center: Challenges and Opportunities
- 1. Department of Pathology, National and Kapodistrian University of Athens, “Attikon” Hospital, 12462 Athens, Greece
- 2. Second Department of Pediatrics, National and Kapodistrian University of Athens, “P. and A. Kyriakou” Children’s Hospital, 11527 Athens, Greece
- 3. Department of Computer Sciences, University of Wisconsin-Madison, Madison, 53706 WI, USA
ABSTRACT
Purpose: The purpose of this paper is to identify the barriers to implementing the national vaccination guidelines and to propose potential intervention strategies to increase vaccination rates in children with rheumatic diseases.
Methods: A cross-sectional descriptive study, conducted in a tertiary hospital in Athens, evaluated the vaccination coverage among 80 pediatric patients with rheumatic diseases, using a 29-item questionnaire. Children up to 16 years of age with delayed or missed vaccine doses were assessed for factors contributing to their incomplete vaccination status.
Results: The overall vaccination rate was 75% (N = 80). The most commonly missed vaccines were the second doses of MMR (15%) and VAR (11%). Based on our age-group classification, children under 2 years of age had missed the DTaP–IPV and HBV vaccine at 6.2%. Among children aged 2 to 6 years, the MMR (17.5%) and VAR (11%) vaccines were most frequently missed. By 10 years of age, the MMR-VAR and HAV vaccines had not been administered in 7.3% and 5% of children respectively, while at 10-12 years, the Tdap and MPV4 vaccines were missed at 28% and 22% of cases. For children ≥ 12 years, HPV (44%) and Tdap vaccines (16%) had been omitted. The COVID-19 vaccinated children were 47% (5 to 12 years old) and 52% (≥ 12 years old). Of all vaccinated children, 41% reported a COVID-19 infection 6-12 months post-vaccination.
Conclusion: The vaccination rates of the Greek pediatric population with rheumatic diseases in our center are adequate, compared to literature reports from other centers.
KEYWORDS
Vaccination Coverage; Rheumatic Diseases; Children; Barriers to Vaccination.
ABBREVIATIONS
DTap: Diphtheria-Tetanus-Acellular Pertussis Vaccine; HAV: Hepatitis A Vaccine; HBV: Hepatitis B Vaccine; HPV: Human Papilloma Virus Vaccine; INFL: Influenza Vaccine; IPVL: Inactivated Poliovirus Vaccine; MMR: Measles-Mumps-Rubella Vaccine; MPV4 (MENVEO):Meningococcal Conjugate Vaccine (covers serogroups A, C, W, Y); PCV-13: Pneumococcal Conjugate Vaccine 13-valent; Tdap: Diphtheria-Tetanus-Acellular Pertussis Adult-type Vaccine; VAR: Varicella Vaccine.
INTRODUCTION
Background
In the 18th century, Jenner initiated the smallpox vaccination, likely without envisioning the disease's disappearance 180 years later. Active immunization induced by vaccinations not only protects the vaccinated individual but also benefits the population as a whole through the phenomenon of herd immunity. Indeed, the collective immunity acts as a protective barrier against the microorganism, and in the presence of adequate number of immune individuals, it can lead to the end of epidemics. Recent measles outbreaks highlight that achieving adequate vaccination coverage remains a global challenge, especially in high-risk populations such as children with rheumatic diseases [1,2]. Due to their underlying condition, that impairs the natural defense mechanisms, and the necessity for long-term immunosuppressive treatment, which may last months or years before being reduced or discontinued, these patients are susceptible to vaccine-preventable diseases.
Immunosuppressive therapy encompasses traditional drugs including glucocorticoids, biologic agents such as Interleukin- 1 Inhibitors (anti-IL1) and Tumor Necrosis Factor Alpha Inhibitors (anti-TNFα), Disease-Modifying Antirheumatic Drugs (DMARDs) like Methotrexate (MTX) and newer such as JAK kinases inhibitors. The risk of bimonthly infection over one year was reported in 57% of nearly 160 children treated with biologic agent for Juvenile Idiopathic Arthritis (JIA). Upper Respiratory Tract Infections (URTIs) were the most frequently reported [3]. Numerous studies confirm higher rates of infections in children receiving anti-TNFα, with a predominance of URTI and varicella co-infection [4-9]. Indeed, the immunosuppressive therapy alters the child's immune response to infections and immunizations, therefore only non-live vaccines are strongly recommended during immunosuppressive therapy. The administration of live vaccines in immunosuppressed patients is tailored individually [10].
Vaccination with Live-Attenuated Vaccines
Live vaccines are generally contraindicated in immunosuppressed patients [11]. However, booster doses for VZV, MMR, and yellow fever can be safely given to children receiving low doses of conventional immunomodulators, as confirmed by the Centers for Disease Control and Prevention [12,13]. When live vaccines are required, the challenge arises due to the necessary minimum 4-week interval between the doses and the initiation of immunosuppressive therapy. In such cases, the interval between doses is extended. Therefore, achieving passive immunization in children with rheumatic disorders depends on both the age at the disease onset or diagnosis and the national vaccination program.
Vaccination with Non-Live Attenuated Vaccines
Inactivated vaccines in children with rheumatic diseases receiving immunomodulatory regimens are not associated with serious adverse effects or relapsed disease, compared to healthy subjects [11]. All non-live vaccines can be administered safely without restriction; however, it is preferable to administer them two weeks prior to treatment to enhance immunogenicity.
Special Circumstances
Live-attenuated vaccines are recommended to be administered 2 weeks before or 3-11 months after intravenous immunoglobulin therapy, depending on the dose given. No data on the immune response of vaccinated children undergoing treatment with anti-CD20 Monoclonal Antibodies (mAbs) is available. It is generally recommended that immunization precedes the mAbs initiation. If the treatment has already begun, inactivated vaccines can be provided after 6 months and live vaccines at least 12 months after treatment [13,14]. Although the immune response is expected to be reduced in individuals receiving B-cell depleting drugs, the seasonal INFL vaccine is recommended, as it primarily stimulates a T-cell-mediated immune response [12].
MATERIALS AND METHODS
Study Design
This cross-sectional study was conducted at the pediatric hospital "Panagiotis and Aglaia Kyriakou" of Athens, evaluating the compliance of the vaccination status of children with rheumatic diseases to the national vaccination program since 2012. Vaccinations were assessed across four age groups: up to 2 years, 2-6 years, 6-10 years and over 10 years old. Data regarding the demographic characteristics, diagnosis, age at diagnosis, treatment types, dosage regimens and treatment duration were extracted rom the participants' registered health records and the medical database of the hospital's Rheumatology Clinic. A questionnaire, designed to assess the parents' beliefs and hesitations concerning vaccines, was also used after obtaining written consent.
Participants
Children up to 16 years old with rheumatic diseases, who were being monitored at the hospital’s Rheumatology Clinic were recruited. Patients without registered medical record of their preceded immunizations were excluded.
Data Sources
The individual health booklet, which was used to verify the vaccinations, the questionnaire completed by parents and the 10-year medical database of the Rheumatology Clinic (from 01/01/2012 to 31/12/2021).
Systematic Errors
The confounding factors included personal reasons for non-vaccination, the children's conditions, their treatment regimens, and age. The latter factor was controlled by stratification. When calculating the number of children vaccinated against COVID-19, those under 5 years of age were excluded. In assessing the correlation of COVID-19 vaccination between patients and their siblings, 34% of children were not included either due to the absence of siblings or because their siblings were over 18 years of age.
Study Size
A total of 120 parents were interviewed, of whom 40 were excluded due to the absence of registered vaccination data.
Statistical Methods
A cross-sectional descriptive study was performed. The chi-square was applied for the correlations studied and Python programming language was used for the data visualization.
RESULTS
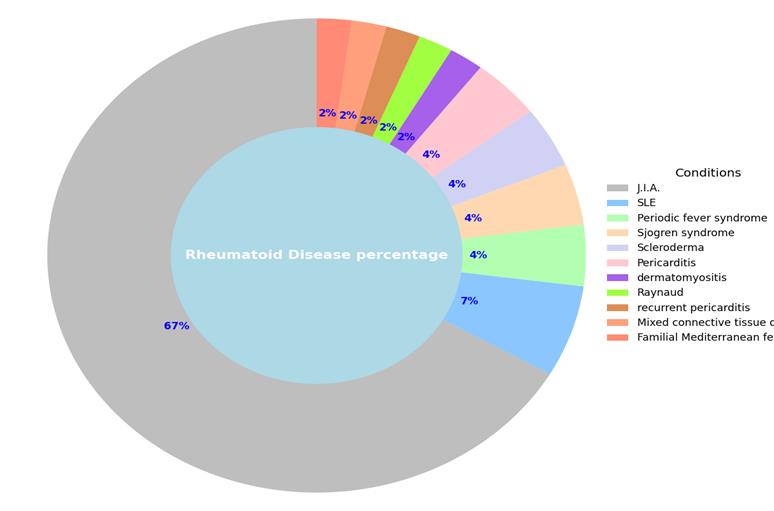
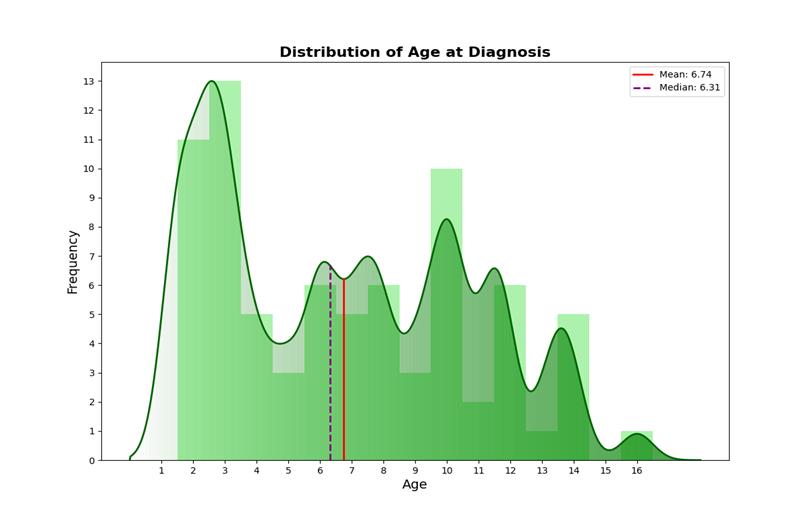
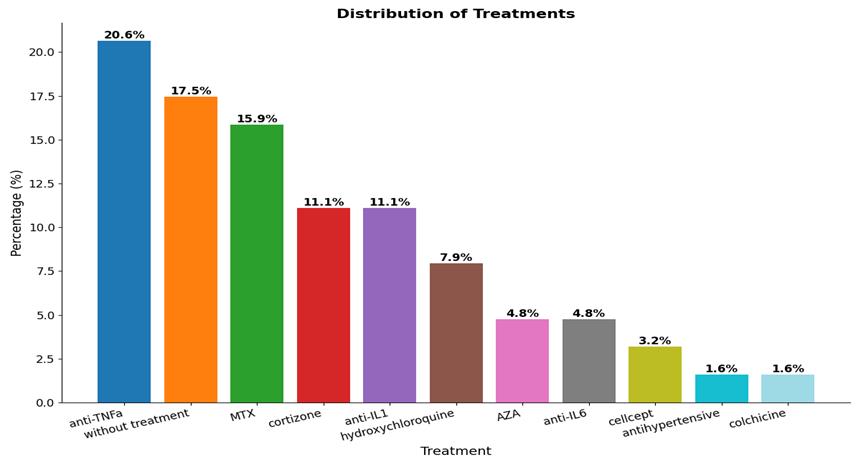
The most common rheumatic disease in the participants was JIA. The overall rheumatic conditions within our study population are summarized schematically (Figure1). The mean age at diagnosis was almost 7 years, with a female-to-male ratio of 2:1 (66.7% vs 33.3%) (Figure 2). Regarding the prescribed treatment, the most frequent regimens were anti-TNFα agents (20.6%), MTX (predominantly at low doses) (15.9%) and anti-IL1 (11.1%) (Figure 3). Based on the questionnaire provided to parents, our conclusions were divided into two categories: overall vaccination coverage and specific coverage for COVID-19.
Figure 1: Distribution of the rheumatic diseases in the study population.
Figure 2: Distribution of age at diagnosis in children with rheumatic diseases.
Figure 3: Distribution of treatments in the study population.
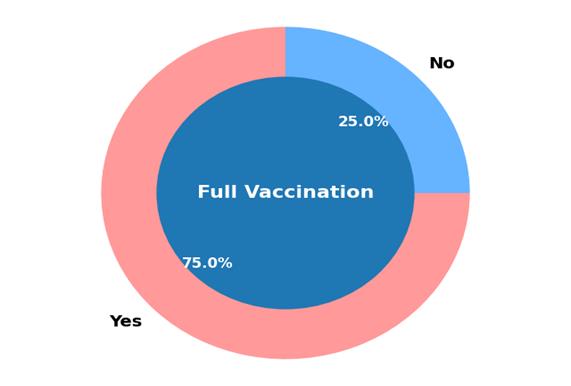
Conclusions on Overall Vaccination Coverage
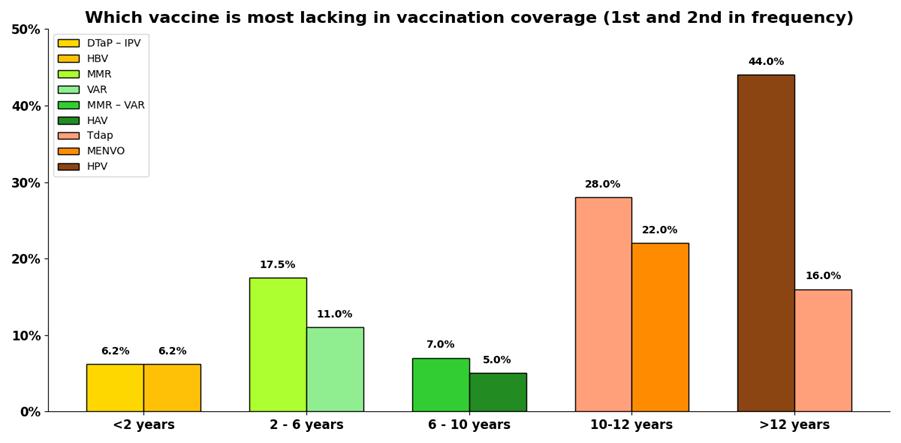
In our study, 25% of the children were not fully vaccinated for their age (Figure 4). The missing vaccines were categorized to predetermined age groups. For children under 2 years old, the DTaP - IPV and HBV vaccines were missing at 6.2% of cases. Among those aged 2 to 6 years, the missing percentages were 17.5% and 11%, but for the MMR and VAR vaccines respectively. In children aged 6 to 10 years, the MMR-VAR and HAV vaccines were administered only at 7% and 5% respectively. From 10 to 12 years old, Tdap and MPV4 vaccines were missing at 28% and 22% of cases, whilst in children aged ≥ 12 years, HPV and Tdap were omitted in 44% and 16% respectively (Figure 5).
Figure 4: Overall vaccination rates in the study population.
Figure 5: Distribution of the missing vaccines per age group in the study population.
In cases of delayed vaccination, we assessed whether the postponement was attributable to national immunization guidelines, erroneous instructions, or factors unrelated to the disease and treatment.Delays due to national guidelines or underlying conditions resulted in postponing the booster doses of VAR and MMR vaccines in 6.5% of cases each. Misguided advice or parental fears led to delays in the second dose of MMR (15%), VAR (15%), and DTaP - IPV (10%). Delays unrelated to disease and treatment were noted for the HBV vaccine in 21% of cases, for the VAR in 13% and for the MMR and the Dtap - IPV vaccine in 11% of cases each. Regarding adverse effects from immunization, 8% of parents reported immunization-related side effects, of these, 50% attributed the onset of JIA to the first dose of the VAR vaccine, while the remaining 50% linked Systemic Lupus Erythematosus (SLE) and rheumatic pericarditis to the COVID-19 vaccine.
???????Conclusions on Vaccination Status for Covid-19
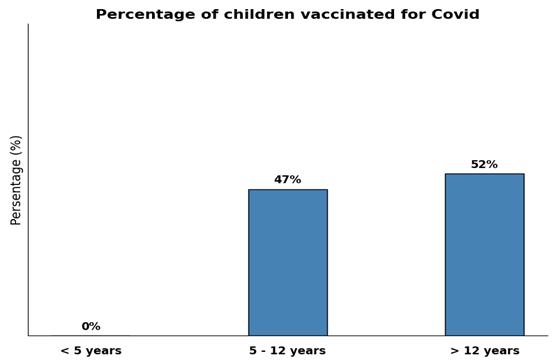
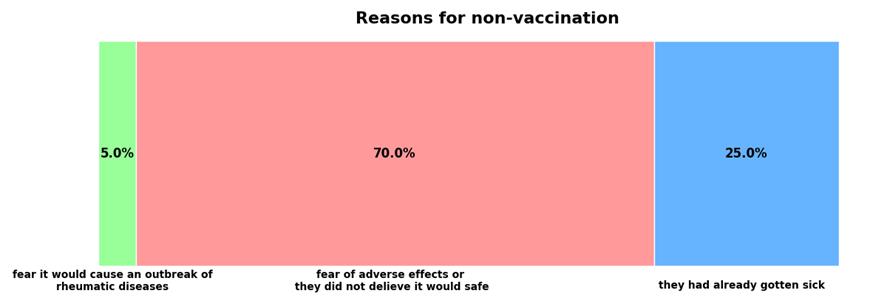
The percentage of children vaccinated for SARS?CoV?2 was 47% for children aged from 5 to 12, and 52% for older children (Figure 6). Potential side effects and vaccine’s safety were the main hesitations of the parents (70%), followed by fears of rheumatic disease relapse post-immunization (5%) (Figure 7). Among the unvaccinated children, 25% had previously contracted COVID-19, and in these cases, the pediatrician did not recommend vaccination. Among the vaccinated children, 41% experienced COVID-19 infection within 6 to 12 months post-vaccination. Although 45% of parents considered their children vulnerable to COVID-19, only 55% of them eventually vaccinated them. The correlation between influenza vaccination and SARS?CoV?2 vaccination raised also concerns in the interpretation of our results. Both infections present with overlapping symptoms, share similar rapid detection methods, and require regular booster vaccine doses. Additionally, co-infection is possible, especially in immunocompromised patients. Of the surveyed patients, 52% were vaccinated for COVID-19 and 45% for influenza, with 64% of the COVID-19-vaccinated also receiving the influenza vaccine, and 74% of the influenza-vaccinated also receiving the COVID-19 vaccine. Using the chi-square test, no statistically significant association was found the two immunizations (χ² = 3.27, DF = 1, a = 0.05,).
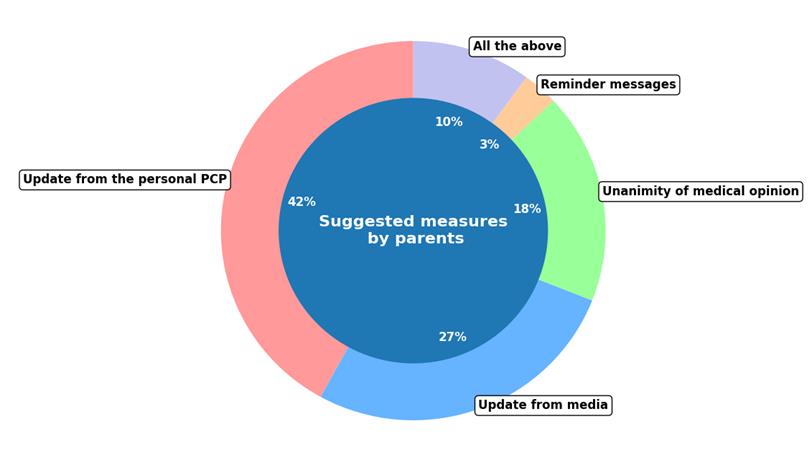
Regarding the siblings of the non-vaccinated for COVID-19 children with rheumatic disease, only a quarter was vaccinated for COVID-19. No statistically significant association was observed between patients unvaccinated for COVID-19 and their vaccinated siblings. Patients without siblings or siblings ≥ 18 years old, were excluded from this analysis (34%). Finally, 83% of parents were consulted on strategies to enhance vaccination rates; the rest 17% did not respond. Concerning the optimal approaches, 42% of parents endorsed the medical updates provided by their pediatrician, while 27% emphasized the influence of the media. Among the interviewed parents, 18% advocated for a consensus among physicians on vaccinations of patients with rheumatic conditions, and 3% recommended text reminders before doses. The remaining 10% believed that all of the aforementioned strategies would be equally beneficial in improving vaccination rates for children with rheumatic diseases (Figure 8).
DISCUSSION
Since children with underlying rheumatic conditions have a compromised natural defense system and receive usually long-term immunosuppressive therapy, they are definitely more vulnerable to infections. Therefore, they should adhere to national vaccination guidelines. In our study, the overall vaccination coverage was 75%, consistent with findings in the literature. In Canada, 40% of children with JIA were not fully vaccinated, according to their country's national immunization schedule [15]. In Ljubljana, only 65% of patients with rheumatic diseases were vaccinated in accordance with the country's national program by age 18, with only 10% having received the seasonal INFL vaccine and 4% PCV-13 [16]. The numbers are even mediocre in a recent observational study in French children with autoimmune diseases, as only 32% had an updated immunization status at age 2, 28% at age 7, 6% at age 15, and 44% at their last outpatient visit. Vaccines recommended for immunosuppressed patients, such as VZV, PCV, and INFL, were also commonly missed, particularly among infants with recurrent febrile episodes [17]. A retrospective case-control study in Turkey further demonstrated significantly lower immunization rates (71.4% versus 95.7%, p < 0.001) between children with rheumatic conditions and healthy controls at the last outpatient visit [18].
Figure 6: COVID-19 vaccination rates per age group in the study sample.
Figure 7: Reasons for non-vaccination as reported by the parents.
Figure 8: Interventive strategies for enhancing the vaccination coverage as suggested by parents.
In our study, multiple vaccines had been omitted in children with rheumatic diseases, including DTaP - IPV and HBV, MMR, VAR, Tdap, MPV4 and HPV. The predominantly missed vaccines were the booster doses of MMR (15%) and VAR (11%), particularly among children aged 2 to 10 years. The same findings arise from literature reports, as only half of the children with recurrent autoinflammatory fever syndromes were fully vaccinated with MMR by age 2 [17]. Similarly, booster doses of MMR and HBV vaccine were frequently missed in children with rheumatic diseases [18]. Data on the administration of a single MMR dose in these children are limited, as the first dose is usually administered before disease onset or diagnosis. Physician’s recommendations were the main reason for delaying immunization in our study: the second dose of MMR was delayed in 15%, the second dose of VAR in 15% and the Dtap - IPV in 10% of cases. Similar rates were found for the second dose of MMR in the Turkish retrospective case-control study, due to medical instructions [18].Given that only 6.5% of our participants were appropriately unvaccinated in accordance with the guidelines, possible vaccine-dropouts could have been reduced, if both paediatricians and rheumatologists had aligned with the recommendations of the Pediatric Rheumatology European Society (PReS), published in 2011 and updated in 2021. Indeed, the intervention of rheumatologists was decisive in increasing vaccinations among children with auto inflammatory fever syndromes during their last visit compared to at age 2. For example, post-intervention immunization rates for PCV-13 were 91% versus 88%, for HBV 79% versus 69%, and for MMR 91% versus 50% [17]. Moreover, healthcare providers frequently delegate vaccination responsibilities to other professionals, overlooking their own critical role in monitoring immunizations.
Delayed doses of live vaccines were unrelated to disease or treatment in 13% of our patients. Despite evidence supporting the safety and immunogenicity of live-attenuated vaccines in children on immunosuppressive therapy, there is still a widespread reluctance towards immunization. This hesitancy is compounded by the fact that most vaccination recommendations for live-attenuated vaccines are based on small cohort studies [19-21]. In our study, half of the parents associated the first dose of the VAR vaccine with the onset of JIA. A recent systematic review of 37 studies involving 2.571 children with Juvenile Autoimmune Rheumatic Diseases (JARD) undergoing immunosuppressive treatment, found ambiguous results regarding disease activity post-vaccination, with no conclusive evidence linking vaccination to disease worsening [22] Indeed, in the literature no serious adverse effects or disease outbreaks have been reported following immunization, except for lower antibody response in children with JARD [22,23], and in adults receiving the yellow fever vaccine [24]. No complicated or disseminated varicella infections occurred after the VZV booster doses in immunosuppressed children [25]. Therefore, live vaccines appear to be safe for children with rheumatic diseases on DMARDs, although they may not be equally immunogenic, potentially requiring additional booster doses in the future [7,22].
The COVID-19 vaccination adherence in our study was 47% for children aged 5 to 12, and 52% for older children. According to our questionnaire, the primary barriers to vaccination were parents' concerns about vaccine safety and potential side effects (70%), pediatricians’ assurances that vaccination was unnecessary due to prior COVID-19 infection (25%) and fears of disease relapse post-immunization (5%). Half of the parents who reported adverse effects following the vaccination, attributed the COVID-19 vaccine to the onset of SLE and pericarditis in teenagers. Conversely, in a retrospective study conducted in Thailand during the COVID-19 pandemic, only 3.36% of patients reported new-onset rheumatic disease following the COVID-19 vaccine. Patients using prednisolone ≥ 10 mg/day before vaccination were more likely to experience a flare [26]. In our study, the underlying disease did not appear to be the primary reason for vaccine hesitancy, as only 25% of the siblings of unvaccinated children with rheumatic diseases were vaccinated for COVID-19. No statistically significant correlation was found between the two groups. Regarding strategies to improve vaccination coverage, the majority of parents (42%) supported mainly the pediatrician's recommendations. This aligns with literature findings encouraging in-depth discussions and communication techniques, such as motivational interviewing, to enhance vaccine acceptance [27].
REFERENCES
- Nelson R. US measles outbreak concentrated among unvaccinated children. Lancet Infect Dis. 2019; 19: 248. doi: 10.1016/S1473-3099(19)30074-X. PMID: 30833064; PMCID: PMC7129894.
- Porter A, Goldfarb J. Measles: A dangerous vaccine-preventable disease returns. Cleve Clin J Med. 2019; 86: 393-398. doi: 10.3949/ccjm.86a.19065. PMID: 31204978.
- Aygun D, Sahin S, Adrovic A, Barut K, Cokugras H, Camc?oglu Y, et al. The frequency of infections in patients with juvenile idiopathic arthritis on biologic agents: 1-year prospective study. Clin Rheumatol. 2019; 38: 1025-1030. doi: 10.1007/s10067-018-4367-9. Epub 2018 Nov 17. PMID: 30448935.
- Toussi SS, Pan N, Walters HM, Walsh TJ. Infections in children and adolescents with juvenile idiopathic arthritis and inflammatory bowel disease treated with tumor necrosis factor-α inhibitors: systematic review of the literature. Clin Infect Dis. 2013; 57: 1318-1330. doi: 10.1093/cid/cit489. Epub 2013 Jul 29. PMID: 23899685; PMCID: PMC3888230.
- Nagy A, Mátrai P, Hegyi P, Alizadeh H, Bajor J, Czopf L, et al. The effects of TNF-alpha inhibitor therapy on the incidence of infection in JIA children: a meta-analysis. Pediatric rheumatology online journal. 2019; 17: 4.
- Lee WJ, Lee TA, Suda KJ, Calip GS, Briars L, Schumock GT. Risk of serious bacterial infection associated with tumour necrosis factor-alpha inhibitors in children with juvenile idiopathic arthritis. Rheumatology (Oxford). 2018; 57: 273-282. doi: 10.1093/rheumatology/kex049. PMID: 28431162.
- Toplak N, Uziel Y. Vaccination for Children on Biologics. Curr Rheumatol Rep. 2020; 22: 26. doi: 10.1007/s11926-020-00905-8. PMID: 32436130.
- Glück T, Kiefmann B, Grohmann M, Falk W, Straub RH, Schölmerich J. Immune status and risk for infection in patients receiving chronic immunosuppressive therapy. J Rheumatol. 2005; 32: 1473-80. PMID: 16078322.
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011; 2011: CD008794. doi: 10.1002/14651858.CD008794.pub2. PMID: 21328309; PMCID: PMC7173749.
- Centers for Disease Control and Prevention (CDC). Contraindications and Precautions. CDC. 2023; 21.
- Heijstek MW, Ott de Bruin LM, Bijl M, Borrow R, van der Klis F, Koné-Paut I, Fasth A, et al. EULAR recommendations for vaccination in paediatric patients with rheumatic diseases. Ann Rheum Dis. 2011; 70: 1704-1712. doi: 10.1136/ard.2011.150193. Epub 2011 Aug 3. PMID: 21813547.
- Groot N, Heijstek MW, Wulffraat NM. Vaccinations in paediatric rheumatology: an update on current developments. Curr Rheumatol Rep. 2015; 17: 46. doi: 10.1007/s11926-015-0519-y. PMID: 26025339; PMCID: PMC4449376.
- Centers for Disease Control and Prevention (CDC). General Best Practice Guidelines for Immunization: Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP) [Internet]. Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html
- Blanchard-Rohner G. Vaccination in Children With Autoimmune Disorders and Treated With Various Immunosuppressive Regimens: A Comprehensive Review and Practical Guide. Front Immunol. 2021; 12: 711637. doi: 10.3389/fimmu.2021.711637. PMID: 34408752; PMCID: PMC8365419.
- Morin MP, Quach C, Fortin E, Chédeville G. Vaccination coverage in children with juvenile idiopathic arthritis followed at a paediatric tertiary care centre. Rheumatology (Oxford). 2012; 51: 2046-2050. doi: 10.1093/rheumatology/kes175. Epub 2012 Aug 3. PMID: 22864995.
- Bizjak M, Blazina Š, Zajc Avramovi? M, Markelj G, Av?in T, Toplak N. Vaccination coverage in children with rheumatic diseases. Clin Exp Rheumatol. 2020; 38: 164-170. Epub 2019 Oct 2. PMID: 31577215.
- Rollet-Cohen V, Mirete J, Dingulu G, Hofer F, Hofer M, Woerner A, et al. Suboptimal vaccination coverage of recommended vaccines among French children with recurrent autoinflammatory fever syndromes: a study from the Juvenile Inflammatory Rheumatism cohort. Clin Rheumatol. 2021; 40: 2855-2864. doi: 10.1007/s10067-020-05553-y. Epub 2021 Jan 13. PMID: 33439385.
- Akgün Ö, Demirkan FG, Kavrul Kayaalp G, Erdemir M, Akay N, Çakmak F, et al. Vaccination coverage of children with rheumatic diseases compared with healthy controls: a retrospective case-control study. Postgrad Med. 2023; 135: 824-830. doi: 10.1080/00325481.2023.2287988. Epub 2024 Jan 10. PMID: 37997766.
- Bijl M, Agmon-Levin N, Dayer JM, Israeli E, Gatto M, Shoenfeld Y. Vaccination of patients with auto-immune inflammatory rheumatic diseases requires careful benefit-risk assessment. Autoimmun Rev. 2012; 11: 572-576. doi: 10.1016/j.autrev.2011.10.015. Epub 2011 Oct 22. PMID: 22037116.
- Borte S, Liebert UG, Borte M, Sack U. Efficacy of measles, mumps and rubella revaccination in children with juvenile idiopathic arthritis treated with methotrexate and etanercept. Rheumatology (Oxford). 2009; 48: 144-148. doi: 10.1093/rheumatology/ken436. Epub 2008 Dec 11. PMID: 19074187.
- Heijstek MW, Ott de Bruin LM, Borrow R, van der Klis F, Koné-Paut I, Fasth A, et al. Vaccination in paediatric patients with auto-immune rheumatic diseases: a systemic literature review for the European League against Rheumatism evidence-based recommendations. Autoimmun Rev. 2011; 11: 112-22. doi: 10.1016/j.autrev.2011.08.010. Epub 2011 Aug 28. PMID: 21896342.
- Keller M, Pittet LF, Zimmermann P. Immunogenicity and safety of routine vaccines in children and adolescents with rheumatic diseases on immunosuppressive treatment - a systematic review. Eur J Pediatr. 2022; 181: 1329-1362. doi: 10.1007/s00431-021-04283-w. Epub 2021 Dec 22. PMID: 34936010; PMCID: PMC8692821.
- Heijstek MW, van Gageldonk PG, Berbers GA, Wulffraat NM. Differences in persistence of measles, mumps, rubella, diphtheria and tetanus antibodies between children with rheumatic disease and healthy controls: a retrospective cross-sectional study. Ann Rheum Dis. 2012; 71: 948-954. doi: 10.1136/annrheumdis-2011-200637. Epub 2011 Dec 15. PMID: 22172491.
- Tonacio AC, do Nascimento Pedrosa T, Borba EF, Aikawa NE, Pasoto SG, Filho JCRF, et al. Immunogenicity and safety of primary fractional-dose yellow fever vaccine in autoimmune rheumatic diseases. PLoS neglected tropical diseases. 2021; 15, e0010002.
- Bizjak M, Heshin-Bekenstein M, Jansen MHA, Ziv A, Angevare S, Uziel Y, Wulffraat NM, Toplak N; PReS Vaccination Working Party. Vaccinology in pediatric rheumatology: Past, present and future. Front Pediatr. 2023; 10: 1098332. doi: 10.3389/fped.2022.1098332. PMID: 36704144; PMCID: PMC9872015.
- Lerkvaleekul B, Charuvanij S, Sukharomana M, Pirojsakul K, Kamolwatwong M, Vilaiyuk S. Outcomes in children with rheumatic diseases following COVID-19 vaccination and infection: data from a large two-center cohort study in Thailand. Front Pediatr. 2023; 11: 1194821. doi: 10.3389/fped.2023.1194821. PMID: 37360372; PMCID: PMC10285492.
- O'Leary ST, Opel DJ, Cataldi JR, Hackell JM; COMMITTEE ON INFECTIOUS DISEASES; COMMITTEE ON PRACTICE AND AMBULATORY MEDICINE; COMMITTEE ON BIOETHICS. Strategies for Improving Vaccine Communication and Uptake. Pediatrics. 2024; 153: e2023065483. doi: 10.1542/peds.2023-065483. PMID: 38404211.