A Non-invasive way to Detect Phospholipid Metabolism of Cancer: In vivo31P-MRS
- 1. Guangdong Provincial People’s Hospital, Zhuhai Hospital, China
Abstract
As we known, many compounds in the human body contain phosphorus which provides vital information on changes in cancer microenvironment. Phosphorous-31 (31P) magnetic resonance spectroscopy (MRS), as a powerful non-invasive tool, can be utilized in diagnosing cancer and monitoring their response to therapy. Thus, it is expected to enable doctors to improve the treatment through personalizing therapy and reducing side effects. This paper presents a review of 31P-MRS as a non-invasive tool to assess unique relevant biomarkers from cancer in patients in vivo clinically.
Keywords
• 31P-MRS; Non-invasive; Cancer
CITATION
Li H (2024) A Non-invasive way to Detect Phospholipid Metabolism of Cancer: In vivo 31P-MRS. J Radiol Radiat Ther 12(1): 1103.
INTRODUCTION
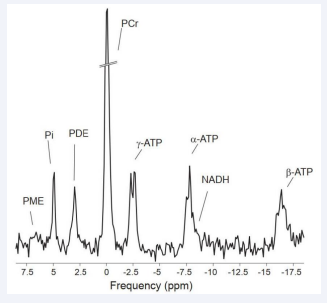
The detection of phospholipid metabolism of cancer in patients is essential for the early diagnosis and treatment monitoring. Magnetic resonance spectroscopy (MRS) is a technique that can complement the latter in providing a wealth of qualitative and quantitative metabolite information of the tissue in question aiding in differentiation between different tumor types and grades [1-4]. 1H has the highest sensitivity which provides high spatial resolution to probe pathological tissue heterogeneity. However, 1H-MRS can monitor aspects of total choline metabolism, it cannot distinguish between multiple phospholipid compounds, such as phosphocholine (PC), phosphoethanolamine (PE), glycerolphosphocholine (GPC) and glycerolphosphoethanolamine (GPE) which can reflect the cell membrane anabolism and catabolism respectively [5,6]. 31P-MRS is probably the most suitable tool to monitor the changes of phospholipid and energy metabolism like phosphocreatine (PCr),inorganic phosphate (Pi) and adenosine triphosphate (α-β-γ-ATP) in human tissue cells ,and also to give important information on intracellular acid levels (PH) (Figure 1) .
Figure 1: 31P-MRS of skeletal muscle. All spectra are depicted relative to the resonance frequency of phosphocreatine (PCr).
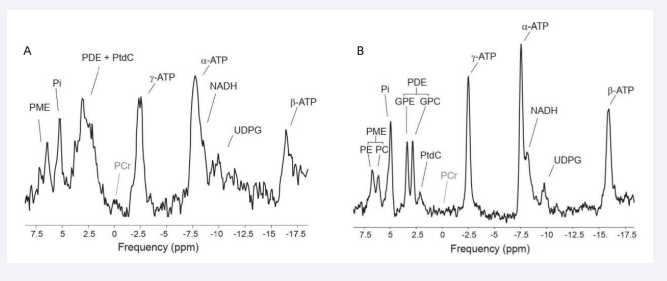
The tumor microenvironment, e.g., tumor vasculature and oxygen consumption, is known to influence choline metabolism. Elevated phosphomonoester (PME) levels (dominated by PC and PE signals) and phosphodiesters (PDE) levels (dominated by GPC and GPE) were observed in malignant tumor through In vivo 31P-MRS studies [4]. Thus, 31P-MRS may become a potential way to give insight into tumor response to therapy. Nevertheless, some previous studies were performed at low magnetic field, leading to insufficient spectral resolution which is unable to detect individual PME and PDE compounds. At higher magnetic field strength, i.e., up to 7T, significantly improved signal-to-noise ratio (SNR) and spectral resolution (Figure 2) were found for in vivo investigations of the human brain [7,8], the human muscle [9-11], and the human breast [12]. This will give in vivo 31P-MRS promising perspective to become a powerful means used in diagnosing cancer and monitoring their response to therapy.
Figure 2: 31P-MRS acquired at 3 T (A) and 7 T (B) in liver tissue where phosphocreatine (PCr) is not present. At 7T, Phosphoethanolamine (PE) and phosphocholine (PC) could be separated from the peak PME, and PDE is readily resolved into glycerophosphorylethanolamine (GPE) and glycerophosphorylcholine (GPC), meanwhile NADPH and UDPG showed up as well.
ALTERATION OF METABOLITES IN CANCER
As is described above, unlike 1H-MR spectra, In vivo 31P-MR spectra have a larger chemical shift range to distribute different resonances. PCR in the majority of human tissue content is relatively high which result in a high intensity peak in the 31P- MR spectrum. Thus, PCr is considered as an internal reference which has a chemical shift of 0 ppm. The relative quantification is applied clinically, that is, the ratio of each metabolite is used for quantitative analysis.
Energy Metabolites
Energy Metabolism of cancer can be assessed through the measurement of bioenergetic metabolites levels which can be identified in 31P-MR spectra (PCr, α-ATP, β-ATP, γ-ATP and Pi). PCR is a storage of high energy phosphate bond which serves as a rapidly available energy reserve in case of increasing energy expenditure. ATP, an important energy source in the metabolism, contains three phosphorous moieties that differ in their resonance frequencies, which can be easily recognized by three individual signals in the 31P-MR spectra. However, in three of them, β-ATP is the most reliable measure of ATP content because both γ-ATP and α-ATP are overlapped with signals from adenosine diphosphate (ADP) and nicotinamide adenine dinucleotide (NADH) [13,14]. With the tumor growth, that PCr and β-ATP resonances decrease while Pi resonance increase is shown in earlier 31P studies [15,16]. When the tumor is growing, development of metabolically active hypoxic cells causes irregular vascular system and insufficient oxygen supply [17].
Phospholipid metabolites
PME (comprising PC and PE) and PDE (mainly GPC and GPE) are known to be cell membrane precursors and degradation products respectively [18,19]. Therefore, PME/PDE ratio is associated with high membrane metabolism turnover rate and studies on the alterations in PME and PDE resonances vary in lots kind of tumors before [20]. An elevation of PME often can be seen in 31P-MR spectra of tumors mainly because enhanced cell membrane synthesis and cell proliferation. Similarly, after proper treatment, a reduction in PME levels was shown in subsequent studies [21,22]. PC and GPC which may relate to enzyme activity had attentions of some other previous studies [4,23-23]. Although 31P-MRS can identify such changes of biomarkers as the cell membrane metabolism altered, uniform standards cannot be determined since the ratio of each phospholipid metabolites can be used as an indicator of treatment monitoring and tumor progression.
Intracellular pH
The resonance positions in 31P-MR spectra of 31P-bearing compounds are defined by their chemical environment, which may change under some particular conditions. Of all the alterations, the effect of pH on inorganic phosphate (Pi) is of particular importance [27]. As noted above, PCr can be seen as a reference since its resonance position is relatively stable. Thus, measured distance between the Pi and the PCr peak(δ) in the spectra can calculate the intracellular pH value [28] with the modified Henderson-Hasselbach equation:
pH = pKa +log [(δ - δHA) / (δA - δ)]
where pKa (6.72) is the dissociation constant of Pi, δHA (3.27) and δA (5.63) are the chemical shifts of the protonated and non-protonated form of Pi respectively. Studies have shown, comparing with normal tissue, acidic extracellular pH and slightly alkaline intracellular pH in tumors [29]. Therefore, the 31P-MRS pH measurement can be an assistant way to detect these variations for diagnosis. Note that Pi or PCr peaks cannot be observed in certain organs, e.g. liver, and other potential candidates should be used [28].
LOCALIZATION TECHNIQUES
Accurate localization technique, which means to acquire the exact signal of VOI (volume of interest) rather than contamination from its outside, is a key for 31P-MRS to apply clinically. Surface coils are often utilized to enhance the poor sensitivity of 31P for spectra [30]. No doubt combining with localization strategy will help to improve accuracy due to some limits of surface coils.
Single voxel localization
There is a variety of single-voxel spectroscopy (SVS) sequences available for traditional 1H localization, for instance, stimulated echo acquisition mode (STEAM) and point resolved spectroscopy (PRESS) [31-33], which are not suitable for 31P metabolites owing to their T2 relaxation times are relatively short. Thus image-selected in vivo spectroscopy (ISIS) [34,35], based on the free induction decay (FID) and had a fast acquisition, is usually preferred for 31P-MRS. It consists of eight scans with different configurations of inversion pulses prior to excitation. Long scan time will be taken in basic ISIS that makes it is sensitive to motion artifacts. Therefore, an improved ISIS sequence was designed to obtain both a clinically feasible measurement time and good spatial resolution [10]. ISIS is a method of choice for transmit surface coils as efficient inversions can be produced with adiabatic RF pulses even in strongly inhomogeneous B1 fields.
Multi-voxel localization
Chemical Shift Imaging (CSI), also called magnetic resonance spectroscopic imaging (MRSI), provides localized NMR spectra in multi pixels of the image. A single slice selective RF pulse is used to excite the imaging slice or a rectangular voxel within the slice. Following the excitation, phase encoding gradients are applied in all spatial dimensions and then the signal is recorded with all gradients off. Producing a spatial metabolite map is main advantage of MSRI, allowing a suitable option [36-38]. However, a high number of phase-encoding steps are required that minimally equals the number of voxels to encode, leading to much longer scan times. For that reason, and due to the low concentration of metabolites observed in in-vivo spectra, CSI is typically used with small matrix sizes. High requirements for magnetic field shimming are needed because MRSI requires a homogeneous main magnetic field over the entire VOI.
TECHNIQUES TO IMPROVE THE QUALITY OF 31P- MR SPECTRA
31P nucleus with low sensitivity makes 31P-MR spectra be subjected to relatively low signal to noise ratio (SNR). Beside high homogeneity of the static magnetic and field high-field strength MR systems, there have been some techniques to enhance 31P sensitivity and improve the quality of the spectrum.
Decoupling
Heteronuclear coupling 1H-31P broadens the resonance lines giving rise to metabolites containing 31P, particularly of PDEs and PMEs compounds, hardly being distinguished. To decouple these affections, RF irradiation should be applied at the 1H frequency using continuous wave or composite pulse during the spectrum acquisition or a part of the spectrum acquisition, gaining higher resolution [39,40]. As a result, separating the metabolites that contribute to overall PDE (GPC and GPE) and PME (PC and PE) signals become possible. When 1H decoupling brings narrow and high spectral lines, it has drawbacks too. With intense RF-pulses, specific absorption rate (SAR) increases, which may be harmful.
Nuclear Overhauser effect
In NMR, if two types of nuclears are close enough spatially, irradiating one of them will increase the signal intensity of another, which is so-called Nuclear Overhauser effect (NOE) being a technique to enhance the signal intensity of low-sensitivity nucleus. In this case, it means to transfer magnetization from 1H to the 31P through irradiation of protons using continuous wave or composite pulse during the repetition time of the sequence. The reported increasing sensitivity of 31P is up to about 80% at lower fields in skeletal muscle [41]. It is noteworthy that NOE is metabolite-specific and depending on magnetic field strength. As well as decoupling, at a price, NOE increases SAR significantly limiting its use at human.
Insensitive nuclei enhanced by polarization transfer
Insensitive nuclei enhanced by polarization transfer (INEPT) is based on the intramolecular scalar coupling (J-coupling) between nuclei which can transfer the polarization of the excited1H spins to the 31P spins. It can provide enhancement of 31P sensitivity up to 50% from the theoretical maximum [42]. For years, many advanced INEPT techniques are designed to gain a better SNR such as refocused insensitive nuclei enhanced by polarization transfer (RINEPT) [43], and the adiabatic version of refocused insensitive nuclei enhanced by polarization transfer (BINEPT) [44]. However, the entire spectrum can be obtained with INEPT due to metabolites, e.g. PME and PDE, with significant 1H-31P J-couplings are suitable for signal enhancement. Therefore, high-energy metabolites cannot be seen in the spectra.
Applications in Cancer
The ability to detect metabolic changes of 31P non-invasively makes 31P-MRS could be essential in the study of cancer. It can describe a tumor’s energetic status and observe alterations in cell membrane phospholipid metabolism, therefore, the biomarkers observed in the spectra are significant for the diagnosis and therapy monitoring of cancer.
Liver
Liver being a highly metabolic organ of human is responsible for many metabolisms, e.g. carbohydrates and lipids. For its importance, hepatic tumors should be treated as soon as possible and have good monitoring of therapy response in order to find appropriate strategy. However, it is difficult to diagnose in the early stage by conventional ways. 31P-MRS give it a chance to make that become reality non- invasively.
The first research about in vivo 31P MR spectra of human liver cancer was in 1985, revealing increased signals of PME which may be considered a diagnostic discriminator [45]. After a successful therapy, studies showed a reduction in PME levels and the spectra became comparable to healthy subjects. Other biomarkers of hepatic infiltration with lymphoma are elevated ratios of PME/ATP and PME/Pi [22]. All of the patients previously diagnosed by conventional ways, e.g. CT, and then elevation of the PME/ATP and PME/Pi ratios were detected in spectra. When successful therapy is done, it makes a decrease in the PME/ ATP ratio, if not, no significant changes were observed in the spectra. Nevertheless, the alterations of these biomarkers are not dependent on the type of tumor which means in vivo 31P-MRS cannot be used to distinguish them.
In order to check whether the resolution of 31P-MRS can be increased to discriminate types of hepatic tumors, experiments by in vitro MRS have been performed. As we mentioned above, PME dominated by PC and PE signals and PDE comprised of GPC and GPE were shown in the spectra. Lower levels of GPC and GPE were observed in hepatic tumors than the healthy ones, and PC and PE were increased. No significant differences between primary and secondary hepatic tumors were observed by in vitro MRS [46].
Breast
Although 31P cannot compete 1H with sensitivity, interestingly the first breast MRS signal was shown in 31P-MRS. Instead of assessing a ‘total choline’ signal that includes Cho PC and GPC in 1H-MRS, 31P-MRS can distinguish between multiple phospholipid compounds, and no water suppression needed. Nowadays, surface coil is widely used in patients with breast tumor to gain the spectra. SNR is usually undesirable owing to motion artifact, e.g. breathing or heart beating. Inhomogeneous B1 providing by surface coil resulting in adiabatic pulses is used for quantitative 31P MR spectroscopy.
Back to 1986, a study on breast cancer was reported by Oberhaensli ?et al., with a 1.9 T scanner [47]. A high PME peak and low PCr were shown in the 31P MR spectrum. After that, the elevated PME were shown in patients comparing with volunteers in another research [48]. These two studies were both localized by surface coil only. A dual-tuned solenoidal coil and ISIS localization were utilized in the following study in 1991, which shown lower (PME+Pi) and higher (PDE+PG) in malignant tumors than benign and healthy tissue. Phosphoglycans (PG) is a signal between PCr and PDE, claimed by the authors [49]. A study of 19 patients with breast cancers in vivo at 1.5 T demonstrated higher PME levels in cancers than healthy ones (p=0.002). And higher ratios of PME/PDE were seen in tumor tissue as compared to healthy tissue (p=0.02), ATP levels were lower on the contrast [50].
During the course of therapy, PME/PDE and PME/Pi ratios decreasing in five breast cancer patients with tumors were reported recently [51]. A dual-tuned quadrature surface coil setup and an adiabatic multiecho spectroscopic imaging AMESING sequence were used at 7.0 T to study prior to, half way and after neoadjuvant chemotherapy.
Brain
31P MRS of 3 brain tumors was obtained before and after radiation or chemotherapy within modified ISIS sequence for localizing. After the therapy, alterations in 31P MR spectra was observed. The tumor spectrum shows that the concentrations of PME were increased and decreased concentrations of PCr [52]. Another paper found that comparing to healthy subjects, decreased levels of PME, PDE, PCr, and ATP in tumors are shown in smaller voxel volumes. The ratios of PCr/Pi, PCr/ATP and ATP/Pi were also found to be reduced in tumors [53]. The pH calculated with the modified Henderson-Hasselbach equation was increased in brain tumors as well. Both experiments are performed with ISIS sequence for localizing.
In 1992, the first study observing a decrease of total 31P signal with MRSI measurements in brain tumors [54]. Following this study, only few papers of 31P MRSI in brain tumor are published in spite of the effort undertaken to show the reproducibility of 31P MRSI in cancer patients within multiple institutions [55]. As the line width in pediatric brain is usually smaller than in adult brain, the spectra were well-resolved allowing quantification of the ratios of PC/GPC and PE/GPE which were found to be significantly higher in primitive neuroectodermal tumors compared to controls. The linewidth is usually smaller than the pediatric brain of adults leading to a good resolution, allowing quantitative PC/GPC and PE/GPE ratios, which were higher in primitive neuroectodermal tumor [56].
With the techniques mentioned above, e.g. PT, high SNR and higher spatial resolution within clinically acceptable measurement times will be acquired to improve the quality of spectra. Application of the selective RINEPT in patients with brain tumors at 3 T was shown in report in order to enhance spatial resolution [57]. Well-resolved signals of PE, PC, GPE and GPC are shown in 31P MR spectra with the selective RINEPT sequence in MRSI mode, allowing to evaluate alterations of the PE/GPE or PC/GPC ratios. As the ultra-high magnetic field improving, e.g. 7T, the SNR of 31P MRS is even more increased. Recently, a direct detection integrated with multi-echo polarization transfer (DIMEPT) acquisition techniques for 31P MRS have been presented to increase in SNR for PMEs in vivo [58].
Prostate
Endorectal coil are used in studies on in vivo 31P-MRS of prostate [59-61]. They have found that the ratios of PME/β-ATP and PME/PCr in prostate cancer are higher than normal tissue, and the ratio of PCr/β-ATP are relatively low at 2 T [60].The low- field strength has its defects that meeting low sensitivity leading to long scan time and poor SNR which are unacceptable clinically. Another consequence of low-field strength is inaccurate spatial localization resulting in neighboring tissue contamination, which makes experimental result distorted. In addition, PME and PDE cannot be resolved into PC, PE and GPC, GPE, which are confirmed in cell cultures and tissue samples to be related to tumor malignancy [62,63].
Therefore, high-field strength (7 T) is widely applied in most of the work in prostate in vivo 31P MRS for optimist SNR and short scan time. However, at high-field strength, the feasibility and safety must be check before it goes to clinical application. T. Kobus Recently et al. have done this work recently at 7 T in healthy human prostate with 31P endorectal coil combined with an eight-channel 1H body-array coil [64]. 3D 31P MRSI was obtained from the whole prostate in 18 min with a relative high spatial resolution. A study based on 15 patients with prostate cancer proven by biopsy at 7 T made comparisons with the results gained in 1990s [65]. As mentioned, increased chemical shift dispersion at 7 T allowed separate detection of PC and PE, and the Pi region was decomposed into two or three separate peaks as well. But high PC and GPC levels only appeared in two patients with high Gleason scores tumors. In low Gleason scores ones no differences of 31P metabolite ratios from healthy tissue, which maybe the reason of the partial volume effects.
Due to the volumes of prostate cancer are usually small, partial volume effects must be avoided. Coming with the resolution-improving techniques is the cost of increased scan time. Therefore, more studies of 31P-MRS in human prostate should focus on high-field strength MR, improved coil setup, and better sequences.
CONCLUSIONS
31P MR spectroscopy and imaging are capable of the noninvasive assessment of phospholipid metabolism of cancer. As the potential of the tool can be more effective at high field strengths, many earlier studies were hampered by low SNR and backward hardware. And with the use of improved techniques, which have been referred to cursorily in the review, e.g. pulse sequence improvement and high-field MR systems etc. To sum up, 31P-MRS is of essentiality in our understanding of phospholipid metabolism of cancer and may be a ideal instrument to diagnosis and monitoring treatment. Nowadays, 31P-MRS is sensitive enough to assess phospholipid metabolic changes clinically despite some defects are still remained to improve in the future.
REFERENCES
- Hollingworth W, Medina LS, Lenkinski RE, Shibata DK, Bernal B, Zurakowski D, et al. A systematic literature review of magnetic resonance spectroscopy for the characterization of brain tumors. AJNR Am J Neuroradiol. 2006; 27: 1404-1411.
- Negendank W. Studies of human tumors by MRS: a review. NMR Biomed. 1992; 5: 303-324.
- Gillies RJ, Morse DL. In vivo magnetic resonance spectroscopy in cancer. Annu Rev Biomed Eng. 2005; 7: 287-326.
- Sharma U, Jagannathan NR. Magnetic Resonance Imaging (MRI) and MR Spectroscopic Methods in Understanding Breast Cancer Biology and Metabolism. Metabolites. 2022; 12: 295.
- Podo F. Tumour phospholipid metabolism. NMR Biomed. 1999; 12: 413-439.
- Glunde K, Jie C, Bhujwalla ZM. Molecular causes of the aberrant choline phospholipid metabolism in breast cancer. Cancer Res. 2004; 64: 4270-4276.
- Lei H, Zhu XH, Zhang XL, Ugurbil K, Chen W. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: an initial experience. Magn Reson Med. 2003; 49: 199-205.
- Qiao H, Zhang X, Zhu XH, Du F, Chen W. In vivo 31P MRS of human brain at high/ultrahigh fields: a quantitative comparison of NMR detection sensitivity and spectral resolution between 4 T and 7 T. Magn Reson Imaging. 2006; 24:1281-1286.
- Bogner W, Chmelik M, Schmid AI, Moser E, Trattnig S, Gruber S. Assessment of (31)P relaxation times in the human calf muscle: a comparison between 3 T and 7 T in vivo. Magn Reson Med. 2009; 62: 574-582.
- Bogner W, Chmelik M, Andronesi OC, Sorensen AG, Trattnig S, Gruber S. In vivo 31P spectroscopy by fully adiabatic extended image selected in vivo spectroscopy: a comparison between 3 T and 7 T. Magn Reson Med. 2011; 66: 923-930.
- Kan HE, Klomp DW, Wong CS, Boer VO, Webb AG, Leijten PR, et al. In vivo 31P MRS detection of an alkaline inorganic phosphate pool with short T1 in human resting skeletal muscle. NMR Biomed. 2010; 23: 995-1000.
- Klomp DW, van de Bank BL, Raaijmakers A, Korteweg MA, Possanzini C, Boer VO, et al. 31P MRSI and 1H MRS at 7 T: initial results in human breast cancer. NMR Biomed. 2011; 24: 1337-1342.
- Irving MG, Simpson SJ, Field J, Doddrell DM. Use of high-resolution 31P-labeled topical magnetic resonance spectroscopy to monitor in vivo tumor metabolism in rats. Cancer Res. 1985; 45: 481-486.
- Evanochko WT, Sakai TT, Ng TC, Krishna NR, Kim HD, Zeidler RB, et al. NMR study of in vivo RIF-1 tumors. Analysis of perchloric acid extracts and identification of 1H, 31P and 13C resonances. Biochim Biophys Acta. 1984; 805: 104-116.
- Okunieff PG, Koutcher JA, Gerweck L, McFarland E, Hitzig B, Urano M, et al. Tumor size dependent changes in a murine fibrosarcoma: use of in vivo 31P NMR for non-invasive evaluation of tumor metabolic status. Int J Radiat Oncol Biol Phys. 1986; 12: 793-799.
- Koeze TH, Lantos PL, Iles RA, Gordon RE. In vivo nuclear magnetic resonance spectroscopy of a transplanted brain tumour. Br J Cancer. 1984; 49: 357-361.
- Rofstad EK, DeMuth P, Fenton BM, Sutherland RM. 31P nuclear magnetic resonance spectroscopy studies of tumor energy metabolism and its relationship to intracapillary oxyhemoglobin saturation status and tumor hypoxia. Cancer Res. 1988; 48: 5440-5446.
- Cohen JS. Phospholipid and energy metabolism of cancer cells monitored by 31P magnetic resonance spectroscopy: possible clinical significance. Mayo Clin Proc. 1988; 63: 1199-1207.
- Daly PF, Lyon RC, Faustino PJ, Cohen JS. Phospholipid metabolism in cancer cells monitored by 31P NMR spectroscopy. J Biol Chem. 1987; 262: 14875-14878.
- Ruiz-Cabello J, Cohen JS. Phospholipid metabolites as indicators of cancer cell function. NMR Biomed. 1992; 5: 226-233.
- Steen RG. Response of solid tumors to chemotherapy monitored by in vivo 31P nuclear magnetic resonance spectroscopy: a review. Cancer Res. 1989; 49: 4075-4085.
- Dixon RM, Angus PW, Rajagopalan B, Radda GK. Abnormal phosphomonoester signals in 31P MR spectra from patients with hepatic lymphoma. A possible marker of liver infiltration and response to chemotherapy. Br J Cancer. 1991; 63: 953-958.
- Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011; 11: 835-848.
- Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999; 59: 80-84.
- Al-Saffar NM, Troy H, Ramírez de Molina A, Jackson LE, Madhu B, Griffiths JR, et al. Noninvasive magnetic resonance spectroscopic pharmacodynamic markers of the choline kinase inhibitor MN58b in human carcinoma models. Cancer Res. 2006; 66: 427-434.
- Esmaeili M, Moestue SA, Hamans BC, Veltein A, Kristian A, Engebraten O, et al. In vivo ³¹P magnetic resonance spectroscopic imaging (MRSI) for metabolic profiling of human breast cancer xenografts. J Magn Reson Imaging. 2015; 41: 601-609.
- Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973; 248: 7276-7278.
- Ackerman JJ, Soto GE, Spees WM, Zhu Z, Evelhoch JL. The NMR chemical shift pH measurement revisited: analysis of error and modeling of a pH dependent reference. Magn Reson Med. 1996; 36: 674-683.
- Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. MRI of the tumor microenvironment. J Magn Reson Imaging. 2002; 16: 430-450.
- Panda A, Jones S, Stark H, Raghavan RS, Sandrasegaran K, Bansal N, et al. Phosphorus liver MRSI at 3 T using a novel dual-tuned eight- channel ³¹P/¹H H coil. Magn Reson Med. 2012; 68: 1346-1356.
- Moonen CT, von Kienlin M, van Zijl PC, Cohen J, Gillen J, Daly P, et al. Comparison of single-shot localization methods (STEAM and PRESS) for in vivo proton NMR spectroscopy. NMR Biomed. 1989; 2: 201- 208.
- Tal A, Gonen O. Spectroscopic localization by simultaneous acquisition of the double-spin and stimulated echoes. Magn Reson Med. 2015; 73: 31-43.
- van Zijl PC, Moonen CT, Alger JR, Cohen JS, Chesnick SA. High field localized proton spectroscopy in small volumes: greatly improved localization and shimming using shielded strong gradients. Magn Reson Med. 1989; 10: 256-265.
- Valkovi? L, Bogner W, Gajdošík M, Povazan M, Kukurova IJ, Krssak M, et al. One-dimensional image-selected in vivo spectroscopy localized phosphorus saturation transfer at 7T. Magn Reson Med. 2014; 72: 1509-1515.
- Bakermans AJ, Abdurrachim D, van Nierop BJ, Koeman A, der Kroon IV, Baartscheer A, et al. In vivo mouse myocardial (31)P MRS using three-dimensional image-selected in vivo spectroscopy (3D ISIS): technical considerations and biochemical validations. NMR Biomed. 2015; 28: 1218-1227.
- Brown TR, Kincaid BM, Ugurbil K. NMR chemical shift imaging in three dimensions. Proc Natl Acad Sci U S A. 1982; 79: 3523-3526.
- Huang Y, Cai S, Zhang Z, Chen Z. High-resolution two-dimensional J-resolved NMR spectroscopy for biological systems. Biophys J. 2014; 106: 2061-2070.
- Tijssen KCH, Bart J, Tiggelaar RM, Janssen J, Kentgens APM, van Bentum PJM. Spatially resolved spectroscopy using tapered stripline NMR. J Magn Reson. 2016; 263: 136-146.
- George C, Chandrakumar N. (1)H NMR with Partial Transition Selectivity. J Phys Chem A. 2022; 126: 314-317.
- Pell AJ, Edden RA, Keeler J. Broadband proton-decoupled proton spectra. Magn Reson Chem. 2007; 45: 296-316.
- Brown TR, Stoyanova R, Greenberg T, Srinivasan R, Murphy-Boesch J. NOE enhancements and T1 relaxation times of phosphorylated metabolites in human calf muscle at 1.5 Tesla. Magn Reson Med. 1995; 33: 417-421.
- Mancini L, Payne GS, Leach MO. Comparison of polarization transfer sequences for enhancement of signals in clinical 31P MRS studies. Magn Reson Med. 2003; 50:578-588.
- Klomp DW, Wijnen JP, Scheenen TW, Heerschap A. Efficient 1H to 31P polarization transfer on a clinical 3T MR system. Magn Reson Med. 2008; 60: 1298-1305.
- Wijnen JP, Jiang L, Greenwood TR, van der Kemp WJ, Klomp DW, Glunde K. 1H/31P polarization transfer at 9.4 Tesla for improved specificity of detecting phosphomonoesters and phosphodiesters in breast tumor models. PLoS One. 2014; 9: e102256.
- Maris JM, Evans AE, McLaughlin AC, D’Angio GJ, Bolinger L, Manos H, et al. 31P nuclear magnetic resonance spectroscopic investigation of human neuroblastoma in situ. N Engl J Med. 1985; 312: 1500-1505.
- Cox IJ, Bell JD, Peden CJ, Iles RA, Foster CS, Watanapa P, et al. In vivo and in vitro 31P magnetic resonance spectroscopy of focal hepatic malignancies. NMR Biomed. 1992; 5: 114-120.
- Oberhaensli RD, Hilton-Jones D, Bore PJ, Hands LJ, Rampling RP, Radda GK. Biochemical investigation of human tumours in vivo with phosphorus-31 magnetic resonance spectroscopy. Lancet. 1986; 2: 8-11.
- Ng TC, Grundfest S, Vijayakumar S, Baldwin NJ, Majors AW, Karalis I, et al. Therapeutic response of breast carcinoma monitored by 31P MRS in situ. Magn Reson Med. 1989; 10: 125-134.
- Merchant TE, Thelissen GR, de Graaf PW, Den Otter W, Glonek T. Clinical magnetic resonance spectroscopy of human breast disease. Invest Radiol. 1991; 26: 1053-1059.
- Twelves CJ, Porter DA, Lowry M, Dobbs NA, Graves PE, Smith MA, et al. Phosphorus-31 metabolism of post-menopausal breast cancer studied in vivo by magnetic resonance spectroscopy. Br J Cancer. 1994; 69: 1151-1156.
- van der Kemp WJ, Stehouwer BL, Luijten PR, van den Bosch MA, Klomp DW. Detection of alterations in membrane metabolism during neoadjuvant chemotherapy in patients with breast cancer using phosphorus magnetic resonance spectroscopy at 7 Tesla. Springerplus. 2014; 3: 634.
- Segebarth CM, Balériaux DF, Arnold DL, Luyten PR, den Hollander JA. MR image-guided P-31 MR spectroscopy in the evaluation of brain tumor treatment. Radiology. 1987; 165: 215-219.
- Hubesch B, Sappey-Marinier D, Roth K, Meyerhoff DJ, Matson GB, Weiner MW. P-31 MR spectroscopy of normal human brain and brain tumors. Radiology. 1990; 174: 401-409.
- Hugg JW, Matson GB, Twieg DB, Maudsley AA, Sappey-Marinier D, Weiner MW. Phosphorus-31 MR spectroscopic imaging (MRSI) of normal and pathological human brains. Magn Reson Imaging. 1992; 10: 227-243.
- Arias-Mendoza F, Payne GS, Zakian KL, Schwarz AJ, Stubbs M, Stoyanova R, et al. In vivo 31P MR spectral patterns and reproducibility in cancer patients studied in a multi-institutional trial. NMR Biomed. 2006; 19: 504-512.
- Albers MJ, Krieger MD, Gonzalez-Gomez I, Gilles FH, McComb JG, Nelson Jr MD, et al. Proton-decoupled 31P MRS in untreated pediatric brain tumors. Magn Reson Med. 2005; 53: 22-29.
- Wijnen JP, Scheenen TW, Klomp DW, Heerschap A. 31P magnetic resonance spectroscopic imaging with polarisation transfer of phosphomono- and diesters at 3 T in the human brain: relation with age and spatial differences. NMR Biomed. 2010; 23: 968-976.
- van der Kemp WJ, Boer VO, Luijten PR, Klomp DW. Increased sensitivity of 31P MRSI using direct detection integrated with multi- echo polarization transfer (DIMEPT). NMR Biomed. 2014; 27: 1248- 1255.
- Kurhanewicz J, Thomas A, Jajodia P, Weiner MW, James TL, Vigneron DB, et al. 31P spectroscopy of the human prostate gland in vivo using a transrectal probe. Magn Reson Med. 1991; 22: 404-413.
- Narayan P, Jajodia P, Kurhanewicz J, Thomas A, MacDonald J, Hubesch B, et al. Characterization of prostate cancer, benign prostatic hyperplasia and normal prostates using transrectal 31phosphorus magnetic resonance spectroscopy: a preliminary report. J Urol. 1991; 146: 66-74.
- Hering F, Müller S. 31P MR spectroscopy and 1H MR imaging of the human prostate using a transrectal probe. Urol Res. 1991; 19: 349- 352.
- Keshari KR, Tsachres H, Iman R, Santos LD, Tabatabai ZL, Shinohara K, et al. Correlation of phospholipid metabolites with prostate cancer pathologic grade, proliferative status and surgical stage - impact of tissue environment. NMR Biomed. 2011; 24: 691-699.
- Komoroski RA, Holder JC, Pappas AA, Finkbeiner AE. 31P NMR of phospholipid metabolites in prostate cancer and benign prostatic hyperplasia. Magn Reson Med. 2011; 65: 911-913.
- Kobus T, Bitz AK, van Uden MJ, Lagemaat MW, Rothgang E, Orzada S, et al. In vivo 31P MR spectroscopic imaging of the human prostate at 7 T: safety and feasibility. Magn Reson Med. 2012; 68: 1683-1695.
- Lagemaat MW, Vos EK, Maas MC, Bitz AK, Orzada S, van Uden MJ, et al. Phosphorus magnetic resonance spectroscopic imaging at 7 T in patients with prostate cancer. Invest Radiol. 2014; 49: 363-372.