An Approach to Radiotherapy Planning in Patients with Cardiac Implantable Electronic Device: Case Report and Review of Literature
- 1. Division of Radiation Oncology, Regional Cancer Centre, India
- 2. Division of Radiation Physics, Regional Cancer Centre, India
Abstract
The incidence of both cardiac disease and cancer is on the rise. With available effective treatment, the life expectancy can be improved in both of these conditions. A significant number of patients are undergoing cardiac defibrillator and pacemaker device implantation, some of them who develop malignancy may require radiation as part of their treatment. These devices can malfunction in the presence of radiation. A prior knowledge of the device, mechanism of interaction with radiation, possible effects and safe approach to treatment is necessary. Here we report a case of patient with implanted defibrillator and radiation treatment along with review of literature.
Keywords
• Pacemaker
• Defibrillator
• Radiation
• Cancer
• Malfunction
Citation
Anjanappa M, Pramod RS, Paramu R, Bhasi S, Menon S, et al. (2016) An Approach to Radiotherapy Planning in Patients with Cardiac Implantable Electronic Device: Case Report and Review of Literature. J Radiol Radiat Ther 4(1): 1057.
INTRODUCTION
The global incidence of cardiovascular disease and cancer has increased over the past decade. Many of the patients with heart diseases have cardiac implantable electronic devices (CIED) to combat pacing and arrhythmia problems. The amalgamation of technology with medical science has helped in treating many complicated disease conditions. Some of these patients may develop malignancy which may require radiotherapy as part of their treatment. It is vital for the treating radiation oncologist, as well as the entire team involved to know the interaction of radiation with CEID, the potential hazard and management of such patients. Here we present our first experience in treating a patient with implantable cardioverter defibrillator.
CASE REPORT
76 year old male patient was evaluated for epigastric discomfort of 2 months duration. He gives history of dull aching non radiating pain in epigastrium, passing dark tarry stools, loss of weight and appetite. Patient gives a past history of cardioverter defibrillator implantation in January 2013. On clinical examination, WHO scale performance status-1 and vital were stable. Abdomen examination and other systems were within normal limits. On Investigation, Upper gastrointestinal endoscopy revealed ulcerated growth in gastric antrum which was biopsy proven carcinoma stomach. Computed tomography(CT) scan of abdomen showed circumferential wall thickening in distal body of stomach with narrowing of pylorus. No obvious infiltration to adjacent structures and no lymphadenopathy. CT Chest did not show any metastases. He underwent total gastrectomy on 11/12/13. The histopathology revealed Mucin secreting adenocarcinoma infiltrating transmurally and duodenum with evidence of lymphovascular emboli(LVE) and perineuralinfiltration(PNI). Four out of 10 lymph nodes show metastases.
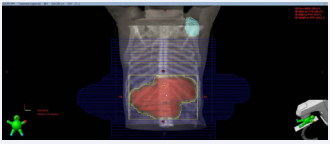
In view of adverse features like node positivity, LVE and PNI the need for adjuvant treatment was explained to the patient and relatives. Adjuvant radiation was alone considered after discussing the benefits and associated risks. Chemotherapy was not given because of cardiac risk and patient request. Before proceeding further, a complete cardiology evaluation was done. A CT simulation was done in the treatment position for radiation planning. The treatment prescription used was 45Gy in 25 fractions (one fraction per day from Monday to Friday) with 3D conformal technique. During planning process, the defibrillator device was contoured to know the dose received (Figure 1).
Figure 1: Beam’s eye view of anterior field showing contoured defibrillator (in cyan) and Planning target volume (in red).
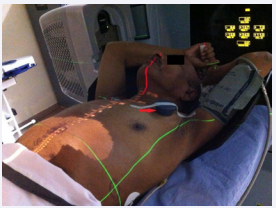
Since the device was not in the treatment field; the planning system calculated dose was zero. Before the start of radiation treatment,the device interrogation was done by the provider to ascertain its functions. A magnet which was provided from the medical device manufacturer was placed over the defibrillator during radiation to disable the “anti-tachycardia mode” without affecting the pacing function (Figure 2).
Figure 2: Patient treatment setup and magnet (arrow) placed over defibrillator.
The distance of the defibrillator from the field edge was 15cm. During the initial 5 fractions of radiation, the dose to the device was monitored by placing optically stimulated luminescent dosimeter (Al2 O3 :C, Landauer,Microstar) over it. From week two onwards dose was monitored once in five fractions using a diode detector (EDD-53G detectors, IBA). The average dose received per fraction was 0. 014Gy. With the collaboration of anesthesia department, electronic monitoring of patient’s pulse, blood pressure andelectrocardiogram was done during treatment for all fractions. A weekly cardiology evaluation was done during the treatment course and on finishing the treatment, device interrogation was repeated. Patient completed the planned course of treatment without any interruptions or events. At one year follow up, patient is asymptomatic and disease free with respect to gastric cancer and the defibrillator is functioning without anomaly.
DISCUSSION
An implantable cardioverter-defibrillator (ICD) is a small battery-powered electrical impulse generator that is implanted in patients who are at risk of sudden cardiac death due to ventricular fibrillation and ventricular tachycardia. The device is programmed to detect cardiac arrhythmia and correct it by delivering a brief electrical impulse to the heart. A pacemaker is a medical device that uses electrical impulses to contract the heart muscles and to regulate the heart rate.
Both the ICD and pacemaker device consists of a pulse generator and batteries. This is usually placed subcutaneously in the left chest below the clavicle. The electrode wires are passed through a vein into the right chamber of heart and the leads are placed in right ventricle. The modern day devices have a complementary metal oxide semiconductor (CMOS) technology. The damage of a pacemaker and ICD during radiotherapy is due to Ionization of semiconductor material and electromagnetic interference from linear accelerators leading to abnormal current flows and changes in threshold voltage [1,2]. The malfunction can also be due to interference with random access memory and reprogramming/resetting of the device [3,4]. Many authors have reported on the effect of radiation on these devices, both clinical data and in vitro studies. The defects range from loss of battery function, resetting of device, erroneous detection of ventricular fibrillation and tachycardia, decreased output, runaway rhythm, sensing defect and inappropriate shock [5-9]. There is no absolute threshold dose for device malfunction and most of the times the exact mechanism of dysfunction cannot be predicted [10]. The tolerance dose to ICD and Pacemaker is different. ICDs are more sensitive to radiation than pacemaker because the operating instructions are stored in the random access memory [11]. The highlights of AAPM recommendations for using radiation in implantable pacemaker are [12].
i) Do not use a betatron
ii) The device should be outside the radiation field,
iii) Dose to the device should be estimated-Total dose should not exceed >2Gy
IV) Cardiology evaluation and patient monitoring before and after radiation.
These recommendations were updated in 1994 in the AAPM TG-34 report [13]. Both of these reports did not mention about monitoring ICD. The dose tolerance to both pacemakers and ICDs varies with wide range [14,15]. There is no uniform recommendation for monitoring ICD and permissible dose limit. In this regard, the manufacturer guidelines should be considered.
Solan et al. has published “universal precautions” in treating patients with ICD or pacemaker [16]. This tries to integrate the AAPM recommendations, manufacturer guidelines and also radiation oncology department policies from various centers. If the cumulative dose exceeds 2 Gy for pacemaker and 1 Gy for ICD, the position of the device should be changed. Although many studies have shown device “body” malfunction, the effect of radiation on the electrodes and lead is largely unknown. It is always safe to follow “As low as reasonably achievable” policy.
A practical guideline proposed by Hurkmanset al. , categorizes patients into low, intermediate and high riskbased on the dose received by the device and pacing dependency [17]. Low risk group include device dose <2Gy and pacing independent. These patients do not require additional measures. Intermediate risk includes pacing dependent group with device dose <2Gy and pacing independent group with device dose 2-10Gy. In these patients, facility for resuscitation, external pacing and monitoring the device weekly should be present. If the device dose is more than 10 Gy, these patients are in high risk group and device relocation should be contemplated and indication for radiation should be re considered. ECG monitoring at each session and device must be checked within 24hrs after each treatment.
Before considering radiation to patients with implantable cardiac devices, the indication for radiotherapy should be reviewed. Relocation of the device should be considered if the device is in the proposed radiation portal. Alternatively radiation fields can be designed to avoid the device if permissible. During treatment planning, the approximate dose received by the device should be calculated.
Since this was our first experience, after a thorough review of literature and guidelines, we formulated a department policy on treating patients with ICD or pacemaker [18-20].
Before Radiation
1. Inform and explain the need for radiation therapy in the present clinical scenario
2. Explain regarding device malfunctioning and complications due to ionizing radiation
3. Obtain high risk consent
4. Detailed Cardiac evaluation
5. Have the ICD/ICP generator moved outside the intended radiation field
6. Complete baseline evaluation of the device
7. Opinion on pacemaker dependent/independent status of the patient and consider deactivation of the device if needed during treatment
Radiation Planning
1. CT simulation as per existing protocol, with inclusion of the device in the imaging
2. Planning of radiation and estimation of dose received to the ICD/ICP
3. Keep the cumulative dose for ICP <2Gy and ICD <1Gy
4. Distance of the device from the radiation field edge should be at least 2. 5cm
Radiation Treatment
1. All personnel involved in the treatment should be notified regarding the device in situ
2. ECG, Blood Pressure and Pulse monitoring before, during and after each treatment session
3. Equipment and medical personnel for resuscitation should be ready
4. Placement of lead shield over the device as indicated by manufacturer
5. Deactivate the device/ placement of magnet for “tachy mode off” if permitted
6. Monitor the dose received by the device using dosimeter
7. Audio Visual monitoring of the patient by Physician during treatment
8. Weekly evaluation by cardiologist and device technician
After treatment
1. Complete evaluation of the device and cardiac functional status by a Cardiologist.
Additional instructions from the manufacturer of the device should be followed:
CONCLUSION
The ICD and pacemaker have different dose tolerance to radiation. Before treatment the guidelines from various sources as well as manufacturer guidelines are to be reviewed. The basic principle of keeping the dose as low as possible is to be followed. Collaboration with the cardiologist and the critical care team is useful in safe delivery of radiation for such patients.
REFERENCES
- Last A. Radiotherapy in patients with cardiac pacemakers. Br J Radiol. 1998; 71: 4-10.
- Niehaus M, Tebbenjohanns J. Electromagnetic interference in patients with implanted pacemakers or cardioverter-defibrillators. Heart. 2001; 86: 246-248.
- Lau DH, Wilson L, Stiles MK, John B, Shashidhar, Dimitri H, et al. Defibrillator reset by radiotherapy. Int J Cardiol. 2008; 130: 37-38.
- Bradley PD, Normand E. Single event upsets in implantable cardioverter de?brillators. IEEE Trans Nuclear Sci. 1998; 45: 2929-2940.
- Uiterwaal GJ, Springorum BG, Scheepers E, De Ruiter GS, Hurkmans CW. Interference detection in implantable defibrillators induced by therapeutic radiation therapy. Neth Heart J. 2006; 14: 330-334
- Katzenberg CA, Marcus FI, Heusinkveld RS, Mammana RB. Pacemaker failure due to radiation therapy. Pacing ClinElectrophysiol. 1982; 5: 156-159.
- Raitt MH, Stelzer KJ, Laramore GE, Bardy GH, Dolack GL, Poole JE, et al. Runaway pacemaker during high-energy neutron radiation therapy. Chest. 1994; 106: 955-957.
- Brooks C, Mutter M. Pacemaker failure associated with therapeutic radiation. Am J Emerg Med. 1988; 6: 591-593.
- Sundar S, Symonds P, Deehan C. Radiotherapy to patients with artificial cardiac pacemakers. Cancer Treat Rev. 2005; 31: 474-486.
- Gelblum DY, Amols H. Implanted cardiac defibrillator care in radiation oncology patient population. Int J RadiatOncolBiolPhys. 2009; 73:1525-1531.
- Guidant Corporation Cardiac Rhythm Management Technical Services. The impact of therapeutic radiation on Guidant implantable pacemakers (IPMs) and implantable cardioverter defibrillators (ICDs), RevisionL02/13/03. 2003: 1-6.
- Marbach JR.Recommended precautions in the management of radiation oncology patients with implanted pacemakers. ASTRO Newsletter. 1989; VIII: 7-8.
- Marbach JR, Sontag MR, Van Dyk J, Wolbarst AB. Management of radiation oncology patients with implanted cardiac pacemakers: report of AAPM Task Group No. 34. American Association of Physicists in Medicine. Med Phys. 1994; 21: 85-90.
- Hurkmans CW, Scheepers E, Springorum BG, Uiterwaal H. Influence of radiotherapy on the latest generation of implantable cardioverter- defibrillators. Int J RadiatOncolBiolPhys. 2005; 63: 282-289.
- Hurkmans CW, Scheepers E, Springorum BG, Uiterwaal H. Influence of radiotherapy on the latest generation of pacemakers. Radiother Oncol. 2005; 76: 93-98.
- Solan AN, Solan MJ, Bednarz G, Goodkin MB. Treatment of patients with cardiac pacemakers and implantable cardioverter-defibrillators during radiotherapy. Int J RadiatOncolBiol Phys. 2004; 59: 897-904.
- Hurkmans CW, Knegjens JL, Oei BS, Maas AJ, Uiterwaal GJ, Van der Borden AJ, et al. Management of radiation oncology patients with a pacemaker or ICD: a new comprehensive practical guideline in The Netherlands. Dutch Society of Radiotherapy and Oncology (NVRO). Radiat Oncol. 2012; 7: 198.
- Wadasadawala T, Pandey A, Agarwal JP, Jalali R, Laskar SG, Chowdhary S, et al. Radiation therapy with implanted cardiac pacemaker devices: a clinical and dosimetric analysis of patients and proposed precautions. Clin Oncol (R Coll Radiol). 2011; 23: 79-85.
- Ferrara T, Baiotto B, Malinverni G, Caria N, Garibaldi E, Barboni G, et al. Irradiation of pacemakers and cardio-defibrillators in patients submitted to radiotherapy: a clinical experience. Tumori. 2010; 96: 76-83.
- Munshi A, Agarwal JP, Pandey KC. Cancer patients with cardiac pacemakers needing radiation treatment: a systematic review. J Cancer Res Ther. 2013; 9: 193-198.