Heterotopic Ossification and Radiotherapy: The Importance of Prophylaxis
- 1. Department of Radiotherapy, Nilratan Sircar Medical College Medical College, India
Citation
Ray A (2025) Heterotopic Ossification and Radiotherapy: The Importance of Prophylaxis. J Radiol Radiat Ther 13(1): 1110.
ABBREVIATIONS
HO: Heterotopic Ossification; RT: Radiation Therapy; BMP: Bone Morphogenetic Protein
INTRODUCTION
Heterotopic ossification (HO) is a pathological condition characterized by the abnormal deposition of mature, lamellar bone within soft tissues where bone formation does not normally occur. Unlike other forms of ectopic mineralization, such as metastatic calcification— often associated with hypercalcemic states-or dystrophic calcification, which arises in necrotic or injured tissues, HO involves the development of structurally organized bone with trabecular architecture. Histologically, HO can be differentiated from dystrophic soft-tissue calcification by the presence of well-defined osteoid formation, Haversian systems, and a distinct trabecular pattern, features that are absent in amorphous calcium deposits. The exact mechanisms driving HO remain an area of active research, but it is known to involve dysregulated osteogenic differentiation of mesenchymal progenitor cells in response to local or systemic triggers [1].
Although the existence of this entity has been known since the turn of the century, diagnostic dilemma coupled with lack of awareness contributes to the inadequate management and ensuing morbidity. This article aims at reviewing the current concepts in pathophysiology and management of HO with special reference to role of radiotherapy in mitigation of HO. This holds an extremely important place in a third world country like India where proper utilization of available resources is a must in health care.
Etiological Classification
HO can be classified a according to the etiology of disease. Accordingly, the causes could be traumatic, neurogenic, and genetic. Traumatic HO occurs following fractures, dislocations, operative procedures, and severe burns. The hip is the most common region of traumatic HO where the process usually involves the abductor compartment. Conversely, burns usually precipitate HO around the elbow joint region.
Neurogenic HO usually occurs following spinal trauma and head injuries, although other central nervous system afflictions like brain tumors, meningitis, encephalitis and subarachnoid hemorrhage have also been implicated. The most commonly involved joint is the hip followed by the shoulder and elbow. Rarely, this condition may result from genetic disorders including fibrodysplasia ossificans progressiva (FOP), progressive osseous heteroplasia (POH), and Albright’s hereditary osteodystrophy (AHO) [1,2] (Table 1).
Table 1: Table presents a short summary of etiology of HO.
|
Acquired |
|
|
Orthopedic procedures, fractures; joint dislocations; or soft-tissue trauma |
|
Hip- usually abductor compartment, Knee, Shoulder, Elbow – commonly after severe burns |
|
|
Hip>shoulder >elbow |
|
Genetic disorders |
|
|
|
Clinical presentation and differential diagnosis
The clinical presentation of heterotopic ossification (HO) is highly variable, with signs and symptoms potentially emerging as early as three weeks or as late as twelve weeks following the inciting event. However, in many cases, HO remains clinically silent and is discovered incidentally during radiographic imaging performed for unrelated reasons [3]. When symptomatic, the most frequent manifestation is restricted joint mobility, which can progress to complete bony ankylosis in severe cases, particularly when HO develops near major joints such as the hip, elbow, or knee. Due to its nonspecific early symptoms-which may include localized pain, warmth, swelling, erythema, and joint stiffness-HO can be easily mistaken for other inflammatory or infectious conditions such as cellulitis, deep vein thrombosis (thrombophlebitis), osteomyelitis, or even neoplastic processes [4].
Fortunately, the majority of HO cases (approximately 80%) follow a benign course without significant progression or long-term functional impairment. However, in the remaining 20% of patients, complications may arise, including persistent joint stiffness, nerve compression syndromes (such as peripheral nerve entrapment), and pressure ulcers due to altered biomechanics. Among these, ankylosis-the fusion of a joint due to excessive bone formation-is the most prevalent serious complication, occurring in roughly 10% of affected individuals [5]. Early recognition and intervention are critical in preventing irreversible disability, though diagnosis in the initial stages remains challenging due to the overlap with other musculoskeletal and inflammatory disorders.
Pathophysiology
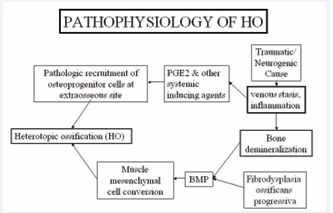
The pathogenesis of heterotopic ossification (HO) is fundamentally rooted in the classic hypothesis proposed by Chalmers et al., which established a tripartite framework for ectopic bone formation [6]. According to this model, three critical elements must converge for HO to develop: (1) the presence of osteoprogenitor cells, which possess the capacity to differentiate into bone-forming osteoblasts; (2) inducing factors, such as bone morphogenetic proteins (BMPs) and prostaglandin E2 (PGE2), which drive osteogenic differentiation; and (3) a conducive local microenvironment that provides the necessary biological signals, including adequate vascular supply, oxygenation, and nutrient availability.
BMPs, particularly BMP-2, -4, and -7, play a central role by activating signalling pathways (e.g., SMAD-dependent mechanisms) that promote mesenchymal stem cell differentiation toward osteoblastic lineages. Meanwhile, PGE2 contributes to the inflammatory milieu that supports osteogenesis, while also modulating pain and vascular responses in the affected tissues. Additionally, mechanical stress, tissue hypoxia, and local immune responses may further influence the progression of HO by altering the extracellular matrix and cytokine profile within the permissive niche (Figure 1).
Figure 1 : Schematic representation of Pathophysiology BMP-bone morphogenetic protein, HO -Heterotopic ossification
Investigations
The diagnostic evaluation of heterotopic ossification (HO) relies heavily on imaging modalities, each offering distinct advantages in detection, characterization, and staging. Conventional radiography (plain X-ray films) remains the initial imaging technique of choice due to its widespread availability and ability to confirm the presence of mature ectopic bone formation. However, while X-rays are typically sufficient for diagnosing established HO, they lack sensitivity in early stages, usually only revealing mineralization 4–6 weeks after the inciting injury [7]. For more precise anatomical localization and assessment of HO extent-particularly in complex anatomical regions or preoperative planning-computed tomography (CT) scans provide superior spatial resolution and three-dimensional reconstruction capabilities. CT is especially valuable in delineating HO proximity to neurovascular structures and adjacent joints [7]. In contrast, isotopic bone scintigraphy (technetium-99m methylene diphosphonate bone scan) offers functional imaging with high sensitivity, detecting early osteoblastic activity as soon as 3 weeks post-injury well before radiographic changes become apparent. This makes it the preferred modality for early diagnosis and monitoring of disease progression [8].
Magnetic resonance imaging (MRI) plays a complementary role, particularly in differentiating HO from other soft-tissue pathologies (e.g., hematomas, infections, or tumors). The classic MRI finding of HO is a peripheral rim of low signal intensity on all pulse sequences, corresponding to the mature bony cortex, with variable central signal reflecting marrow elements. [9] Advanced sequences (e.g., fat-suppressed T2-weighted or gradient-echo imaging) may further characterize perilesional edema or inflammatory changes. While MRI is less specific than CT for mature HO, it excels in evaluating early inflammatory phases and soft-tissue complications.
This tiered imaging approach-combining functional (bone scan), structural (X-ray/CT), and soft-tissue (MRI) modalities—allows for stage-appropriate diagnosis and management optimization [7-9].
The typical radiological appearance of HO is circumferential ossification with a lucent center. Conventional radiographs have been used to classify HO that develops after total hip arthroplasty as shown in Table 2.
Table 2: Brooker’s Grading scale for HO around hip [10].
|
Grade 0 No bone islands visible |
|
Grade 1 Islands of bone visible within soft tissue about the hip |
|
Grade 2 Bone spurs from pelvis or proximal end of femur, leaving >1 cm between opposing surfaces |
|
Grade 3 Like Grade II, except that space between opposing surfaces is <1 cm |
|
Grade 4 Apparent bony ankylosis |
Clinicoradiological classification of elbow HO is presented in Table 3.
Table 3: Graham and Hastings classification of functional limitation caused by heterotopic ossification of the elbow [11].
|
Class |
Description |
|
I |
Radiographic evidence of heterotopic ossification without functional limitation |
|
IIA |
Limitation in elbow flexion/extension |
|
IIB |
Limitation in forearm pronation/supination |
|
IIC |
Limitation in flexion/extension and pronation/supination |
|
III |
Ankylosis of the forearm, elbow, or both |
Bone Scintigraphy in Heterotopic Ossification
Three-phase technetium-99m bone scintigraphy represents the gold standard for early detection and longitudinal monitoring of heterotopic ossification (HO), offering unparalleled sensitivity in identifying ectopic bone formation during its initial metabolic phase. This modality detects osteoblastic activity up to 2.5 weeks post-injury-nearly a month before radiographic changes become apparent—through characteristic findings in each phase:
1) Flow phase (Angiographic phase): Demonstrates increased perfusion to the affected soft tissues, reflecting early hypervascularity associated with osteogenic precursor cell recruitment.
2) Blood pool phase: Shows persistent tracer accumulation, indicating ongoing inflammation and early matrix mineralization.
3) Delayed phase (3-4 hours post-injection): Reveals intense focal uptake correlating with active bone formation, typically positive by 3.5 weeks post-injury. [12]
Beyond diagnosis, serial quantitative bone scans provide critical prognostic information. The technique’s ability to:
- Track metabolic activity trends through standardized uptake value (SUV) measurements
- Identify peak osteoblastic activity (typically 6-12 weeks post-injury)
- Document metabolic quiescence (mandatory before surgical intervention)
makes it indispensable for surgical planning. Studies demonstrate that resection performed during active mineralization phases carries up to 80% recurrence risk, whereas delaying excision until scintigraphic normalization (usually ≥6 months) reduces recurrence to <10% [13]. Recent advances like SPECT/CT fusion imaging further enhance localization accuracy while maintaining scintigraphy’s functional assessment capabilities [12,13].
Differential Diagnosis and Diagnostic Challenges in Heterotopic Ossification (HO) Imaging
The imaging differentiation of heterotopic ossification requires careful distinction from several clinically significant conditions, including neoplastic, infectious, inflammatory, and vascular pathologies. The characteristic temporal progression, histological composition, and distinct radiographic patterns of HO serve as key discriminators from these alternative diagnoses
Neoplastic Conditions: HO must be distinguished from primary bone tumors such as osteosarcoma and benign lesions like osteochondromas. While both HO and osteosarcoma demonstrate new bone formation, HO typically develops more rapidly (weeks to months versus months to years) and lacks the aggressive periosteal reaction, cortical destruction, and soft tissue invasion characteristic of malignant bone tumors. Osteochondromas, while also demonstrating mature bone, maintain continuity with the underlying medullary canal - a feature absent in HO.
Infectious and Inflammatory Conditions:
The diagnostic challenge is particularly significant in differentiating HO from:
1. Cellulitis and thrombophlebitis (especially in immobilized patients)
2. Osteomyelitis
3. Septic arthritis
The potential for HO to cause external vascular compression further complicates the evaluation of suspected deep venous thrombosis, necessitating careful vascular assessment in high-risk patients [14]
Nuclear Imaging Considerations:
The differentiation from osteomyelitis presents unique challenges, as both 67Gallium and, less commonly, 111Indium-labeled white blood cells may show uptake in immature HO. Several distinguishing features aid in differentiation:
- The 67Ga/99mTc-diphosphonate uptake ratio is typically lower in HO than in osteomyelitis
- HO demonstrates characteristic anatomical predilection for proximal joints (hips, knees, shoulders, elbows)
- Bilateral involvement is common in HO (seen in 60 70% of cases)
- Multi-joint region involvement frequently occurs [15]
Diagnostic Algorithm:
A systematic approach combining:
1. Temporal progression analysis
2. Anatomical pattern recognition
3. Multi-modality correlation (radiographs, CT, MRI, scintigraphy)
4. Quantitative nuclear medicine interpretation greatly enhances diagnostic accuracy. The characteristic zonal maturation pattern of HO (peripheral ossification progressing centrally) on CT and MRI provides additional differentiation from neoplastic and infectious processes [14,15].
Management of Heterotopic ossification
Prophylaxis or early treatment of HO is extremely important due to the significant morbidity associated with the condition. Complications of HO include peripheral nerve entrapment, pressure ulcers, and functional impairment if joint ankylosis develops.
For patients in whom clinically significant HO has already developed, management includes passive range of-motion exercises to maintain joint mobility and surgical excision. Criteria for surgical excision are presented in Table 4.
Table 4: Criteria for surgical excision [16].
|
1. Significantly limited range of motion for involved joint |
|
2. Absence of local fever, swelling, erythema, or other clinical findings of acute heterotopic ossification |
|
3. Normal serum alkaline phosphatase |
|
4. Return of bone scan findings to normal or near normal; if serial quantitative bone scans are obtained, there should be a sharply decreasing trend followed by steady state for 2–3 mo. |
Surgical removal of HO is followed by prophylactic measures to prevent recurrence. HO prophylaxis is also performed when there are other indications of high risk for HO development, such as when there is a prior history of HO or after acetabular fracture. The two primary prophylactic modalities are radiation therapy (RT) and nonsteroidal anti-inflammatory drugs (NSAIDs), most commonly indomethacin [17]. In the past, bisphosphonates were also used for prophylaxis [18].The limited durability of this approach coupled with the inevitable recurrence of HO after stoppage of medication has prompted the stoppage of the use of this medication in management of HO [19].
Historical Evolution and Current Standards of Radiation Therapy for Heterotopic Ossification Prophylaxis
The scientific foundation for using radiation therapy (RT) in heterotopic ossification (HO) prevention originates from mid-20th century animal studies that demonstrated radiation’s capacity to impair bone mineralization, with the degree of inhibition being directly related to the timing of administration following bone injury [20]. These findings were complemented by clinical observations of radiation’s effects on developing skeletal systems. Neuhauser et al.’s landmark 1952 study first quantified this phenomenon, showing that radiation doses exceeding 20 Gy could significantly impair bone growth in pediatric patients, particularly when administered to very young children during active skeletal development [21].
Building upon these principles, Coventry and Scanlon conducted pioneering clinical work in 1981 that established RT as a viable HO prevention strategy. Their protocol employed 20 Gy delivered in 10 fractions to high-risk patients following hip surgery, with treatments initiated anywhere from immediately postoperative to 69 days after surgery. The study’s parallel-opposed field technique, covering the ipsilateral hip and proximal femur, yielded a 19% incidence of severe HO - a marked improvement over historical controls. While not statistically powered to demonstrate optimal timing, their observation of better outcomes with earlier treatment initiation laid the groundwork for subsequent timing investigations. This represented the first successful clinical application of RT for HO prophylaxis [22].
The 1980s saw significant protocol refinements aimed at optimizing the risk-benefit ratio:
1. Dose Reduction: Sylvester et al.’s pivotal 1988 study demonstrated equivalent efficacy between 10 Gy and the original 20 Gy regimen, while potentially reducing long term risks [23].
2. Timing Optimization: Subsequent research established the critical 72-96 hour postoperative window for treatment initiation, corresponding to the peak of mesenchymal cell differentiation.
3. Fractionation Schemes: Healy et al.’s work in the early 1990s validated single-fraction regimens (7-8 Gy) as equally effective as multifraction courses, offering practical advantages [24].
Modern protocols have evolved through multiple randomized trials to current standards:
-Dose:7-8 Gy single fraction or 10 Gy in 2-5 fractions
-Timing: Within 72 hours postoperatively (ideally <24 hours)
- Target Volume: Soft tissues surrounding high-risk joints
- Technique: Advanced modalities like IMRT/VMAT to minimize scatter dose [25].
This progression reflects an improved understanding of radiobiological effects on osteoprogenitor cells, the inflammatory cascade preceding ossification and practical considerations in postoperative care. Contemporary studies continue to refine ultra-hypofractionated regimens, combined modality approaches (RT + NSAIDs), and Image guided techniques for precision and long-term outcome monitoring. The historical evolution from 20 Gy/10fx to current protocols demonstrates successful translation of radiobiological principles into clinically effective, patient friendly regimens [20-25].
The narrow therapeutic window for effective postoperative radiation therapy in preventing heterotopic ossification presents several significant clinical challenges that have prompted investigation into alternative timing strategies. The immediate postoperative period creates particular difficulties due to multiple factors. Patients often experience significant postoperative pain that limits their mobility and ability to maintain proper positioning during radiation treatment. Furthermore, restricted weight-bearing status following procedures like total hip arthroplasty complicates patient transportation and setup. There is also the potential for surgical complications such as wound drainage or hematoma formation that may delay radiation administration beyond the optimal therapeutic window.
These practical challenges, combined with important theoretical considerations regarding the biology of heterotopic ossification, led researchers to explore preoperative radiation therapy. The target mesenchymal progenitor cells responsible for ectopic bone formation primarily reside in periarticular soft tissues and maintain their osteogenic potential immediately before surgery. These radiosensitive cells become activated within hours of surgical trauma, suggesting that preoperative radiation could be equally effective at preventing their differentiation into bone-forming cells.
This biological rationale, supported by promising animal studies demonstrating equivalent efficacy of preoperative radiation, led Gregoritch and colleagues to conduct a pivotal randomized clinical trial comparing preoperative versus postoperative radiation protocols in high-risk patients undergoing total hip arthroplasty. Their study enrolled patients with either a history of heterotopic ossification or ankylosing spondylitis, randomizing them to receive either a single 7-8 Gy fraction within four hours before surgery or the same dose within 72 hours after surgery. Radiation was delivered through a single anterior posterior field covering the periacetabular soft tissues and proximal femur, with careful shielding of prosthesis components.
The trial results demonstrated no significant difference in outcomes between the two approaches. The overall incidence of heterotopic ossification was 26% in the preoperative group compared to 28% in the postoperative group. More importantly, the rates of clinically significant heterotopic ossification (Brooker grade III/IV) were 2% versus 5% respectively, suggesting a potential advantage for preoperative administration in preventing severe cases. The study found no differences in wound complications between groups and equivalent functional outcomes at twelve-month follow-up.
These findings established several important clinical principles. First, they confirmed that the critical radiobiologic targets remain equally vulnerable to radiation immediately before surgical activation. Second, preoperative administration avoids many of the logistical challenges associated with postoperative delivery. The slightly lower incidence of severe heterotopic ossification in the preoperative group may reflect more effective cell cycle arrest before surgical trauma activates the osteogenic pathway. Importantly, neither timing approach was found to negatively affect short-term surgical outcomes or prosthesis stability.
This paradigm shift in treatment timing offered several practical advantages for clinical practice, including simplified patient logistics through single hospitalization, reduced treatment delays from postoperative complications, potential cost savings from combined care episodes, and maintained prosthesis dosimetry without increased loosening risks. Subsequent studies have reinforced these findings while further clarifying the optimal preoperative timing window (2-4 hours before incision) and demonstrating equivalent long term recurrence rates beyond five years. Current clinical guidelines now endorse preoperative radiation therapy as an equivalent alternative to postoperative administration, particularly for patients with anticipated mobility challenges, cases where postoperative pain control may be difficult, or in centers with limited postoperative radiation access [26].
Radiotherapy versus Indomethacin
Indomethacin is commonly used for prophylaxis, given its ease of administration and low cost. It is typically given over a period of 5–6 weeks at 25 mg three times per day. However, prophylaxis with indomethacin is not without drawbacks. First, many patients find it difficult to comply with the prescribed treatment course. Second, prolonged use of NSAIDs is associated with gastrointestinal side effects, such as gastritis and ulcer formation. Gastrointestinal bleeding is of particular concern because these patients require deep vein thrombosis prophylaxis with warfarin or low molecular-weight heparin. Finally, indomethacin has been found to increase the rate of bone non-union after fracture. A number of Phase III trials have been conducted comparing these two approaches and meta-analysis of seven such randomized studies demonstrated RT to be more effective than NSAIDs in preventing clinically significant (Brooker Grade 3 or 4) HO [19].
Side effects of Radiotherapy
The feared complication of radiation to bone is development of second malignancies. However, given the minimum latency of 10 years for second malignancy development and the lack of reported cases in the literature, RT seems a rational and safe approach for these patients. Other problems such as trochanteric non union and oligospermia can easily be prevented using appropriate shielding measures [1].
CONCLUSION
Single fraction of external beam radiotherapy can serve as an effective modality for prevention of HO. The low cost of treatment coupled with ease of timing and delivery of treatment makes radiotherapy an attractive modality. Moreover, recent data showing superiority of Radiotherapy over indomethacin-based prophylaxis serves to strengthen the position of this oft ignored modality. Proper identification and prophylaxis can lead to a better quality of life in the subgroup of patients presenting at risk for development of HO.
REFERENCES
- Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: Pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys. 2006; 65: 1289-1299.
- Garland DE. A clinical perspective on common forms of acquired Heterotopic ossification. Clin Orthop Relat Res. 1991; 13-29.
- Wharton GW, Morgan TH. Ankylosis in the paralyzed patient. J Bone Joint Surg Am. 1970; 52: 105-112
- Orzel JA, Rudd TG. Heterotopic bone formation: clinical, laboratory, and imaging correlation. J Nucl Med. 1985; 26: 125-132.
- Brooke MM, Heard DL, de Lateur BJ, Moeller DA, Alquist AD. Heterotopic ossification and peripheral nerve entrapment: early diagnosis and excision. Arch Phys Med Rehabil. 1991; 72: 425-429.
- Chalmers J, Gray DH, Rush J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br. 1975; 57: 36-45
- Bressler EL, Marn CS, Gore RM, Hendrix RW. Evaluation of ectopic bone by CT. AJR. 1987; 148: 931-935
- Suzuki Y, Hisada K, Takeda M. Demonstration of myositis ossificans by 99mTc pyrophosphate bone scanning. Radiology. 1974; 111: 663-664.
- De Smet AA, Norris MA, Fisher DR. Magnetic resonance imaging of myositis ossificans: analysis of seven cases. Skeletal Radiol. 1992; 21: 503-507.
- Brooker AF, Bowerman JW, Robinson RA, Riley LH Jr. Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973; 55: 1629-1632.
- Hastings H 2nd, Graham TJ. The classification and treatment of Heterotopic ossification about the elbow and forearm. Hand Clin. 1994; 10: 417-437.
- Tyler JL, Derbekyan V, Lisbona R. Early diagnosis of myositis ossificans with Tc-99m diphosphonate imaging. Clin Nucl Med. 1984; 9: 256-258.
- Tanaka T, Rossier AB, Hussey RW, Ahnberg DS, Treves S. Quantitative assessment of para-osteo-arthropathy and its maturation on serial radionuclide bone images. Radiology. 1977; 123: 217-221.
- Nagaraj N, Elgazzar AH, Fernandez-Ulloa M. Heterotopic ossification mimicking infection: scintigraphic evaluation. Clin Nucl Med. 1995; 20: 763-766.
- Güngör F, Yazici M, Egehan I, Demirçali AE, Sahin M, Cakir S, et al. Thallium-201 uptake in myositis ossificans. Potential pitfall in diagnosis. Clin Nucl Med. 1995; 20: 259-262.
- Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002; 43: 346-353.
- Neal BC, Rodgers A, Clark T, Gray H, Reid IR, Dunn L, MacMahon SW. A systematic survey of 13 randomized trials of non-steroidal anti- inflammatory drugs for the prevention of heterotopic bone formation after major hip surgery. Acta Orthop Scand. 2000; 71: 122-128.
- Thomas BJ, Amstutz HC. Results of the administration of diphosphonate for the prevention of heterotopic ossification after total hip arthroplasty. J Bone Joint Surg Am. 1985; 67: 400-403
- Pakos EE, Ioannidis JP. Radiotherapy vs. nonsteroidal anti- inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: A meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2004; 60: 888-895.
- Craven PL, Urist MR. Osteogenesis by radioisotope labeled cell populations in implants of bone matrix under the influence of ionizing radiation. Clin Orthop Relat Res. 1971; 76: 231-233.
- Neuhauser EB, Wittenborg MH, Berman CZ. Irradiation effects of roentgen therapy on the growing spine. Radiology. 1952; 59: 637- 650.
- Coventry MB, Scanlon PW. The use of radiation to discourage ectopic bone. A nine-year study in surgery about the hip. J Bone Joint Surg Am. 1981; 63: 201-208.
- Sylvester JE, Greenberg P, Selch MT, Thomas BJ, Amstutz H. The use of postoperative irradiation for the prevention of heterotopic bone formation after total hip replacement. Int J Radiat Oncol Biol Phys. 1988; 14: 471-476.
- Lo TC, Healy WL, Covall DJ, Dotter WE, Pfeifer BA, Torgerson WR, et al. Heterotopic bone formation after hip surgery: Prevention with single-dose postoperative hip irradiation. Radiology. 1988; 168: 851-854.
- Healy WL, Lo TC, DeSimone AA, Rask B, Pfeifer BA. Single-dose irradiation for the prevention of heterotopic ossification after total hip arthroplasty. A comparison of doses of five hundred and fifty and seven hundred centigray. J Bone Joint Surg Am. 1995; 77: 590-595.
- Gregoritch SJ, Chadha M, Pelligrini VD, Rubin P, Kantorowitz DA. Randomized trial comparing preoperative versus postoperative irradiation for prevention of heterotopic ossification following prosthetic total hip replacement: Preliminary results. Int J Radiat Oncol Biol Phys. 1994; 30: 55-62.