Is One Hydrogen Magnetic Resonance Spectroscopy Needed in Diagnostic Evaluation of Children with Developmental Delay?
- 1. R4 Resident, Department of Radiology, King Abdulaziz University Hospital, Kingdom of Saudi Arabia
- 2. Neuroradiology Assistant Professor. Department of Radiology, King Abdulaziz University Hospital, Kingdom of Saudi Arabia
- 3. Radiological Technologist, Department of Radiology, King Abdulaziz University Hospital, Kingdom of Saudi Arabia
- 4. Department of Internal medicine, king Faisal Specialist Hospitals and Research Center, Kingdom of Saudi Arabia
Abstract
Background: Developmental delays in children may result from a variety of neurological adverse effects on the developing brain. One of the most important methods for assessing children with developmental delay is neuroimaging, specifically magnetic resonance imaging (MRI) of the brain, which provides useful information about brain tissue structure and abnormalities. In this study, we aim to evaluate the utility of MRS in the diagnostic evaluation of children with developmental delays who have normal brain structures.
Methods: This is a retrospective study done at King Abdulaziz University Hospitals. We included 70 patients who have undergone structural MRI brain and spectroscopy done in the same setting, their ages ranged from 3 days to 21 years. MR spectroscopy was performed in all patients by using a single voxel point-resolved spectroscopy (PRESS).
Results: A total of Seventy patients were studied from 2015 – 2024. The results indicate that all patients who had normal structural MRI also had normal spectroscopy findings. However, of the 11 participants who had MRIs suggestive of metabolic pathology, only 2 children (15.40%) showed no evidence of metabolic disease on subsequent spectroscopy imaging.
Conclusions: We suggest performing MRI/1HMRS only if there is an MRI brain structural abnormality. Otherwise performing MRI spectroscopy in patients with normal structural MRI has no added value.
Keywords
• Developmental delay
• Magnetic resonance imaging (MRI)
• Magnetic resonance spectroscopy (MRS)
• Point-Resolved Spectroscopy (PRESS)
Citation
Algarni A, Ghoneim A, AL-Juhani A, Ghoneim S (2025) Is One Hydrogen Magnetic Resonance Spectroscopy Needed in Diagnostic Evaluation of Children with Developmental Delay? J Radiol Radiat Ther 13(2): 1113.
ABBREVIATIONS
DD: Developmental Delay; FLAIR: Fluid Attenuated Inversion Recovery; MRS: Magnetic Resonance Spectroscopy; PRESS: Point-Resolved Spectroscopy; SWI: Susceptibility-Weighted Imaging; TR: Repetition Time; WM: White Matter; CR: Creatine; DWI: Diffusion Weighted Imaging; MRI: Magnetic Resonance Imaging; NAA: N-acetyl aspartate; SPSS: Statistical Package for the Social Sciences; TE: Echo Times; WHO: World Health Organization; CHO: Choline
INTRODUCTION
Developmental delay (DD) is the result of multiple neurological adverse effects in the developing brain.About 5% of the world’s children at 14 years old have childhood disability or DD, according to the World Health Organization (WHO) [1]. There are many etiologies for developmental delay, including Metabolic Deficiencies, Toxins Infestations, Genetic syndromes, Environmental Factors and some of which cannot be identified without neuroimaging, including the level of peri-natal hypoxic injury and structural brain abnormalities [2,3]. One of the most important methods for assessing children with developmental delay is neuroimaging, specifically magnetic resonance imaging (MRI) of the brain, which provides useful information about brain tissue structure and abnormalities. Magnetic resonance spectroscopy is a non-invasive method for measuring the concentrations of various metabolites in brain tissue [4]. Recent research suggests that second-line neuroimaging methods using challenging MRI protocols, such as proton magnetic resonance spectroscopy, can be helpful for children with DD and normal MRI results (MRS) especially if applied specifically to patients with neurological signs such as abnormal head circumference (microcephaly, non-familial macrocephaly, rapid change in head circumference ), focal neurological signs or epilepsy, abnormalities of the white matter (WM), abnormal appearance of the cerebellum, whereas its role is very limited in children without these signs [5,6]. Also, previous studies have reported that MR Spectroscopy in children with normal MRI brains revealed no significant difference in the neurometabolite ratios [7]. The presence and ratios of different metabolites quantified by MRS provide Strikethrough information on the assessed brain parenchyma. For children with DD, Studies have shown that NAA levels are reduced in conditions associated with neuronal damage or neuronal loss [8,9]. MRS is effective in detecting hypoxic-ischemic encephalopathy and mitochondrial encephalopathies, metabolic causes, and neoplasms [10,11]. Previous studies have reported variations in NAA/Cr and the Cho/Cr ratio in abnormal myelination [12,13]. There is a paucity of data regarding the value of MRS in children with DD who were found to have a normal appearance of the brain parenchyma on the conventional MRI sequences. In this study, we aim to evaluate the utility of MRS in the diagnostic evaluation of children with developmental delays who have normal brain structures.
METHODOLOGY
Patient
This is a retrospective study done in the Department of Radiology at King Abdulaziz University Hospital. This study was approved by the hospital’s ethics committee (reference number 573-22). Written patient consent was waived.We assessed the MRI examinations performed for children and young adults; their ages ranged from 3 days to 21 years. We included 70 patients who have undergone structural MRI brain and spectroscopy done in the same setting for investigation of a clinically suspected metabolic brain disease. We excluded patients who had MRI spectroscopy done for tumor or infection investigation.
Neuroimaging
All MRI examinations were performed in a 3 tesla (Siemens magnetom verio M. No. 10185400, made in Germany), scanner equipped with 20-channel head coil.
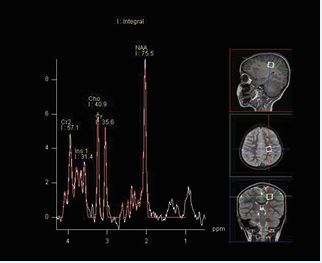
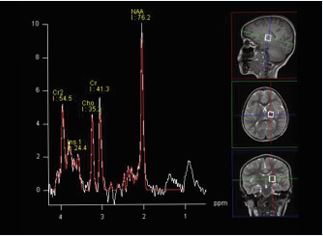
MRI technique: The protocol consisted of axial T1, axial FLAIR, axial DWI, axial SWI, coronal T2, and sagittal T1 sequences. Proton MR spectroscopy was performed in all patients by using a single voxel point-resolved spectroscopy (PRESS) sequence with a repetition time (TR) of 1500 ms and two echo times (TE) of 144 milliseconds (ms) and 35 ms, voxel size of 15 mm were used. As illustrated in Figure A and B, two locations were assessed with both the long and short TE MRS. One in the frontoparietal deep white matter and the other in the basal ganglia. The metabolites assessed include NAA, choline, creatine, myo-inositol, and lipids/lactate. All MRI examinations were reviewed by an experienced pediatric neuroradiologist.Data were coded and recorded in an Excel spreadsheet program. Statistical Package for the Social Sciences (SPSS) software, Version 27.0.0.0 (IBM Corp., Armonk, NY) was used for data analysis. A descriptive analysis of the experience of the participating groups was provided.
Figure A: Single-voxel PRESS acquisition in the frontoparietal deep white matter.
Figure B: Single-voxel PRESS acquisition in the basal ganglia.
RESULTS
Patients
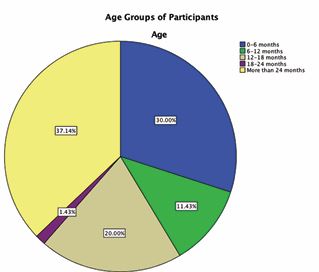
We collected a total of 70 patients from 2015 to 2024. 57.10% of participants were male (N=40) while 42.90% were female (N=30). Our patients varied in age from 0-6 months to older than 24 months. The majority (37.14%) were older than 24 months of age. The least age group to have participated are those between the ages of 18 and 24 months (1.43%). A more detailed description of participants’ ages can be found in Figure C.
Figure C: Pie chart demonstrating age groups of participants.
Structural MRI and Spectroscopy
The majority (81.40%), of participants included in this study had structural MRIs free from evidence of metabolic disease (57 participants). The remaining 18.60% had MRIs suggestive of metabolic abnormalities. After undergoing spectroscopy imaging, 84.30% were found to have no evidence of metabolic disease, while 15.7% showed metabolic abnormalities on spectroscopy. Of those who had no evidence of metabolic abnormality on MRI, 100% were found to have no evidence of metabolic disease on spectroscopy. Meanwhile, of the 11 participants who had MRIs suggestive of metabolic pathology, only 2 children (15.40%), showed no evidence of metabolic disease on subsequent spectroscopy imaging. There is a statistically significant relationship between both structural MRI findings and spectroscopy as p=0.000 as per Chi-square analysis and Fisher’s exact test. The attached Table 1 demonstrates the discussed frequencies of normal and abnormal metabolic findings on the two imaging modalities.
Table 1: Cross-tabulated Frequencies of Metabolically Normal and Abnormal Imaging.
|
|
Spectroscopy |
|
|
|
Structural MRI |
Normal |
Abnormal |
Total |
|
Normal |
57 |
0 |
57 |
|
Abnormal |
2 |
11 |
13 |
|
Total |
59 |
11 |
70 |
DISCUSSION
The results indicate that all patients who had normal structural MRI also had normal spectroscopy findings. On the other hand, of the 11 participants whose MRI scans suggested metabolic disease, only 2 children (15.40%) showed no evidence of metabolic disease on subsequent spectroscopic imaging. These findings are consistent with the result obtained by Vasantha et al [14], showing that MR Spectroscopy in children with normal MRI revealed no major difference in the neuro metabolite ratios. On the other hand of patients who had MRIs suggestive of metabolic abnormalities only 15.7% showed metabolic abnormalities on spectroscopy and these results are in concordance with a lot of studies focused on the value of MRI spectroscopy in patients with abnormal structural. H-MRS may be of diagnostic value in certain cases with characteristic metabolic patterns. However, there are some limitations to our study. It was conducted at a single medical center, which may limit the generalizability of the findings. Differences in patient demographics, clinical protocols, and technological variations across multiple centers could affect the reproducibility of results. Additionally, the study population may not be large enough to account for all potential variations in metabolic profiles among children with developmental delay. A larger cohort from multiple institutions could strengthen statistical power and provide a broader perspective. We believe our findings may significantly influence current clinical practice by potentially reducing overall scan time and MRI related costs if applied effectively. Based on our results, we suggest performing MRI combined with ¹H-MRS only in cases where structural brain abnormalities are identified. Otherwise, spectroscopy appears to offer no added value in patients with normal MRI findings.
CONCLUSION
The findings of this study suggest that MRI/1HMRS should be conducted exclusively when a structural abnormality is identified in the brain MRI. Performing MRI spectroscopy in cases where the structural MRI appears normal does not provide additional diagnostic value. Therefore, a targeted approach to MRI spectroscopy is recommended to optimize clinical utility and resource allocation.
AUTHOR CONTRIBUTIONS
Ayshah and Alia were involved in the conception of the article, performed the literature search and data analysis, and drafted and critically revised the manuscript. Aliaa and Solafa performed the data analysis. Ayshah and Abdullah collected the data and drafted and critically revised the manuscript. All the authors have reviewed and approved the final manuscript.
ACKNOWLEDGEMENT
The authors express their gratitude to King Abdulaziz University Hospital, Jeddah, Saudi Arabia.
REFERENCES
- Developmental Delay / Delayed Milestones. 2025.
- Bélanger SA, Caron J. Evaluation of the child with global developmental delay and intellectual disability. Paediatrics Child Health. 2018; 23: 403-410.
- Evaluation of Pediatric Patients with Developmental Delay: a Cross- Sectional Study. Cureus. 2022.
- Momen AA, Jelodar G, Dehdashti H. Brain magnetic resonance imaging findings in developmentally delayed children. Int J Pediatr. 2011; 2011: 1-4.
- Verbruggen KT, Meiners LC, Sijens PE, Lunsing RJ, Van Spronsen FJ, Brouwer OF. Magnetic resonance imaging and proton magnetic resonance spectroscopy of the brain in the diagnostic evaluation of developmental delay. European J Paediatr Neurol. 2008; 13: 181-190.
- Mithyantha R, Kneen R, McCann E, Gladstone M. Current evidence- based recommendations on investigating children with global developmental delay. Arch Dis Childhood. 2017; 102: 1071-1076.
- Gupta R. A study of magnetic resonance imaging and spectroscopy ofbrain in children with developmental delay. 2021; 7: :329-334.
- Yeo RA, Hill D, Campbell R, Vigil J, Brooks WM. Developmental Instability and Working Memory Ability in Children: A Magnetic Resonance Spectroscopy Investigation. Dev Neuropsychol. 2000; 17: 143-159.
- Ford CC, Griffey RH, Matwiyoff NA, Rosenberg GA. Multivoxel 1 H-MRS of stroke. Neurology. 1992; 42: 1408-1418.
- Rama Jayasundar, Singh VP, Raghunathan P, Jain K, Banerji AK. Inflammatory granulomas: evaluation with proton MRS. NMR Biomed. 1999; 12: 139-144.
- Horská A, Barker PB. Imaging of Brain Tumors: MR Spectroscopy and Metabolic Imaging. Neuroimaging Clinics of North America. 2010; 20: 293-310.
- Moore GJ. Proton magnetic resonance spectroscopy in pediatric neuroradiology. Pediatric Radiology. 1998; 28: 805-814.
- Gupta R. A study of magnetic resonance imaging and spectroscopy of brain in children with developmental delay. 2021; 12: 329-334.