Tirzepatide in Obstructive Sleep Apnea: A Comprehensive Review of Its Role and Impact
- 1. Doctor of Pharmacy, PES University, Banashankari, Bengaluru, India
Abstract
Background: Obstructive Sleep Apnea (OSA) is a prevalent long-term condition that has important metabolic effects. Through weight loss, better glycemic management, and other mechanisms, tirzepatide, a dual GLP-1 and GIP receptor agonist, has been demonstrated to be useful in controlling OSA.
Objective: The purpose of this review was to explore the impact and potential of tirzepatide in OSA management, including clinical evidence, advantages over the current therapy and future applications.
Methods: A comprehensive review of recent clinical trials, literature including SURMOUNT- OSA and mechanistic studies.
Results: Tirzepatide significantly reduces the Apnea-Hypopnea Index (AHI) which is a measuring parameter of OSA, improves metabolic parameters, and offers a treatment option for patients who cannot adhere to the traditional standard therapy.
Conclusion: Tirzepatide represents a breakthrough in OSA treatment, warranting further exploration in long-term trials.
Keywords
- Obstructive Sleep Apnea (OSA)
- Tirzepatide
- GLP-1 receptor agonist
- GIP receptor agonist
- SURMOUNT-OSA Trial
- Apnea Hypopnea Index (AHI)
- Metabolic dysfunction
- Weight management
- Insulin resistance
- Glycemic control
CITATION
Bhat B, Nikitha AS (2025) Tirzepatide in Obstructive Sleep Apnea: A Comprehensive Review of Its Role and Impact. J Sleep Med Disord 9(1): 1144.
INTRODUCTION
OSA is defined by classical signs of slumber breathing disorder resulting from the repetitive episodes of airway obstruction which causes lowering of oxygen saturation.OSA has always been a tricky disease to treat. Excessive daytime tiredness, apnoeas during sleep, and loud, disturbing snoring are further signs of OSA [1]. A recent development in the field of treatment can give people who are battling these disorders fresh hope [2]. The FDA has approved tirzepatide as a treatment for chronic weight management in adults who are obese (BMI ≥ 30), or overweight (BMI ≥ 27) with at least one weight-related health condition like high blood pressure, type 2 diabetes or high cholesterol [3,4].
Epidemiology of OSA and its Link with Diabetes
There is a strong Bidirectional relationship between OSA and diabetes. Studies have shown that OSA is 83% more prevalent in Type 2 Diabetes patients. The mechanism linking the OSA and diabetes include intermittent hypoxemia, sleep fragmentation which are pivotal players in causing metabolic dysfunction. These features not only define OSA but also play a role in metabolic issues. Recent studies have shown that the severity of OSA independently correlates with insulin resistance in people who don’t have type 2 diabetes. This link describes not only how OSA has an effect on sleep but it also has an impact on the metabolic system in the body [5,6].
Mechanism of Tirzepatide in OSA
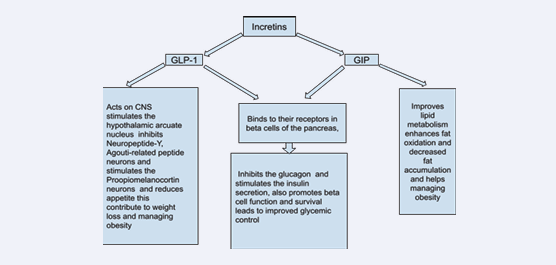
The tirzepatide stimulates the receptors of GLP-1 and GIP hormones and results in decreased appetite. Tirzepatide has two polypeptide chains: the first of these is modeled on GLP-1 and the second, which is GIP homologous. The outcomes of the tests imply that it binds to the GLP-1 and GIP receptors and activates the receptors and produces effects similar to the effects of endogenous incretins. Among them, one is an analogue of GLP-1 and the other is an analogue of GIP. It bindings to the GLP-1 and GIP receptors and activates the receptors and causes mimicking the actions of incretins that are found naturally. Therefore, incretins are endogenous hormones required in the regulation of glucose homeostasis [7]. Two mainly known incretins are: Glucagon-Like Peptide 1 (GLP-1) and Glucose-Dependent Insulinotropic Polypeptide (GIP). Incretins are released by L-cells in the gastrointestinal tract in response to the consumption of food, especially carbohydrates and fats. Once in circulation, incretins go to various target organs like the pancreas where glucose dependent stimulation of insulin secretion occurs.
This is useful in lowering high blood glucose level [8,9]. The high GI induced GIP activates the GIP receptors of the pancreatic beta cells which in turn increase intracellular cAMP and calcium. Moreover, GIP is important in increasing the beta cells proliferation, as well as fat metabolism [10]. The GLP-1 binds to GLP-1 receptors produces the same glycemic impact on the pancreas. GLP-1 also affects the rate of gastric emptying, blocks food intake and restricts glucagon secretion that helps in regulation of glucose. Also, it has a number of effects: GLP-1 increases insulin secretion, inhibits the glucagon secretion, prevents beta cell destruction, and promotes their replication.
It is widely accepted that incretins play a role in regulation of appetite and satiety via many targets. When used, GLP-1 enhances satiety and significantly suppresses appetite, which in one way or the other reduces food consumption. It was also previously found that incretins have effects on the vagus nerve of CNS and slow down the emptying in gastric chambers along with the onset of the feeling of satiety. In addition to effects on adipocyte development, viability, and differentiation, GIP may also facilitate a reduction in body weight and healthy expansion of white adipose tissue thereby decreasing development of ectopic fat stores which helps improve insulin sensitivity [9] (Figure 1).
Figure 1: Functions of GLP-1 and GIP.
Tirzepatide in OSA
Conventional treatment of OSA has been Continuous Positive Airway Pressure (CPAP) therapy, which has been known to suffer from compliance problems with patients [10,11]. But the latest clinical trial revealed the tirzepatide which can be used to treat OSA and does not cause the patient compliance problems which is seen in the traditional approach. It may be the easier option for persons who cannot take or follow conventional therapies used in managing the OSA [7]. The complex effect of tirzepatide implies anti-inflammatory effect and modulation of metabolism through enhanced secretion of insulin and better glycaemic control in patients with obesity and overweight. The tirzepatide has been said to be an ideal drug candidate for treatment of OSA due to the multiple mechanisms of action.
Tirzepatide particularly affects metabolic dysfunction and it promotes glucose-stimulated insulin release [12]. That is why it addresses some of the potential fundamental problems connected with OSA. It also increases the quality of sleep disturbances that are among the major complaints presented by OSA patients. It reduces sleep disturbances, as insulin resistance is stated to increase sleep disturbances and the drug refines and enhances the functioning of insulin, correcting the resistance [13,14]. It can alleviate the sleep disturbance mostly by enhancing glycaemic control and insulin signalling.
Weight Management: Tirzepatide and similar GLP-1 receptor agonists have helped lose weight by mechanisms such as appetite suppression and delayed gastric emptying [12]. Obesity is a leading factor to OSA since there is a propensity for fat build up round the airway in the final analysis leads to the blocking of the airway during sleep [13].
Inflammatory Pathways: Tirzepatide may also act on inflammatory processes involved in OSA. Experiments have shown that GLP-1 receptor stimulation could be anti- inflammatory and lessen the chronic inflammation that causes upper airway hypofunction and hypoxic episodes [15].
Neurological Effects: The same receptor is present in many copies in the central nervous system, particularly in structures linked to sleep and breathing [16].The particular effects on neural substrates involved with OSA are under investigation.
Clinical Trial Evidence
The Apnea Hypopnea Index is the common standard for measuring sleep apnea severity. The results of SURMOUNT- OSA showed an improvement in the Apnea Hypopnea Index, a measure of sleep apnea severity compared to placebo. The secondary outcomes like hypoxic burden, systolic Blood Pressure, C - reactive protein levels and patient reported symptoms also significantly improved. There were 2 arms in the study. Study 1 included the patients of OSA who were not on Continuous positive airway pressure and study 2 included patients who were receiving Continuous positive airway pressure.
The study reported a mean reduction of up to 62.8% on the Apnea-Hypopnea Index (AHI) or about fewer events restricting or blocking a person’s airflow per hour of sleep compared to placebo. In a key secondary endpoint showed that 43.0% of the subjects in study 1 and 51.5% of the subjects in study 2 who are treated with tirzepatide with the highest dose attained an AHI of less than 5 events per hour or an AHI of 5-14 events per hour and an Epworth Sleepiness Scale (ESS) score of ≤ 10. Epworth sleepiness scale is a standard questionnaire designed to assess excessive daytime sleepiness. In SURMOUNT-OSA Study for both study 1 and Study 2 the maximum tolerated dose of tirzepatide given was 10 mg or 15 mg once weekly. They started with a dose of 2.5 mg of tirzepatide then increased by 2.5 mg every four weeks until maximum tolerated dose was achieved. 15 mg of tirzepatide was the maximum dose for participants who tolerated 15 mg.
Participants who tolerated 10 mg but did not tolerate15 mg maintained on 10 mg as their maximum tolerated dose [17]. The study conducted by Atul Malhotra, et al., involved 469 participants with moderate-to-severe OSA and clinical obesity. Over 52 weeks, participants were given either tirzepatide or a placebo. The results showed a significant reduction in the number of breathing interruptions during sleep for those who took tirzepatide. This improvement was much greater than in the placebo group, and some participants even reached a point where Continuous Positive Airway Pressure (CPAP) therapy might not be necessary [7].
Navigating Safety: Contextual Overview of Treatment Options Including Tirzepatide in Obstructive Sleep Apnea
Possible unwanted effects may affect the gastrointestinal tract and the symptoms of the side effects may include nausea, diarrhoea, vomiting, and constipation, Some of the severe side effects that may arise, list of the severe sideffects that the users experience when using the drug includes the following: Kidney complications that involves the problem of the gallbladder and inflammation of the pancreas commonly referred to as pancreatitis. It also has cautions about possible thyroid tumours. In summary, Tirzepatide is efficient in treating diseases, such as obesity and OSA. It is crucial to analyze both typical and potentially fatal side effects during its administration [18].
Contraindications: It is contraindicated in Medullary Thyroid Carcinoma, Multiple Endocrine Neoplasia type 2, severe sensitivity to the drug or ingredient, severe GI disorders and pregnancy, breastfeeding. Further research is needed when it is applied to the pediatric and geriatric individuals [19].
Pharmacokinetics
Absorption: Relative bioavailability of tirzepatide is about 80 percent. The time to reach maximum serum concentration varies from 8 hours to 72 hours.
Distribution: Overall, steady-state volume of distribution is 10.3 L for tirzepatide and it is 99% bound to the plasma albumin.
Metabolism: The peptide structure of tirzepatide undergoes proteolytic cleavage to produce smaller peptide fragments. C20 fatty diacid attached to the part of tirzepatide undergoes β-oxidation and further undergoes amide hydrolysis, which aids in its breakdown. After these processes, tirzepatide further breaks down into amino acids in various tissues especially in the liver.
Elimination: Tirzepatide has a five-day half-life permitting predictable once-weekly dosing, and metabolites are excreted in urine and feces [17].
Positioning Tirzepatide: Advantages over Current Therapies and Future Directions
Continuous positive airway pressure (CPAP) provides a constant stream of air at a specific pressure to the airways throughout inspiration and expiration. The most likely side effects of CPAP are nasal congestion, runny nose, drooling, or bleeding gums. These could be eased by using a humidifier with the CPAP. Possible side effects of using a mask were rhinitis, conjunctivitis, and irritation or redness of the face due to continuous pressure. These can generally be avoided by using an appropriate size and padding of the mask so that it does not cause excessive pressure on the skin leading to pressure sores. While there are many advantages of CPAP therapy, adherence to it whether at home, in the hospital, or other healthcare settings remains a major obstacle to its effectiveness.
Most of these patients use the devices for a short time because they are uncomfortable. Continuous positive airway pressure in the treatment of obstructive sleep apnea does not diminish the risk of cardiac event [20]. Several previous studies have shown that CPAP treatment for OSA significantly increases the weight and BMI of patients. In obese or overweight OSA patients, additional weight loss therapy should be considered after initiating CPAP treatment [21].
Improved Cardiometabolic Outcomes
A number of studies have shown that Obstructive Sleep Apnea (OSA) is related to many cardiovascular diseases including atrial fibrillation, coronary artery disease, heart failure, stroke, and hypertension. In addition, those suffering from OSA tend to have a worse prognosis for Cardiovascular Disease (CVD) and a higher future chance of developing it. OSA is rather common, affecting as many as 40-60% of individuals who have CVD and also affecting 34% of men in the generalized population and 17% of women [22].
The SURMOUNT-1 trial showed that tirzepatide could help decrease cardiometabolic threats in non-diabetic obese people. Together, the dual receptor agonism enhances insulin secretion, anti-inflammatory responses, the lipid profile and maintains endothelial integrity. Compared with placebo, tirzepatide might serve as a comprehensive technique in patients with both type 2 diabetes and obesity to improve CV factors [23].
In addition to reductions in OSA severity, the significant reduction in BMI and favorable changes in blood pressure and inflammation profile point to broader health benefits of tirzepatide. This could perhaps lead to a decline in other diseases that are linked with OSA such as hypertension, heart disease, and diabetes [18].
Combination Therapy Potential
Tirzepatide in addition to CPAP therapy may benefit OSA patients with regard to cardiometabolic risk factors and the severity of the disease. It may have the most value for patients with nonintervention emergent CPAP treatment or those which need further therapeutic intervention.
Long-Term Evaluation
Subsequent phase III trials will be vital in determining how long the Tirzepatide effects sustain OSA and weight related changes and if it can minimize the CVD risk. Recognising the long-term successes would be imperative in the integration of tirzepatide in the large OSA management approach. Tirzepatide might be especially helpful for the patient who experiences poor compliance with CPAP use or who wants to try for a better less invasive treatment option of OSA [7].
Tirzepatide demonstrated significant improvements when used in combination with CPAP therapy which is supported by the evidence from the trial. Tirzepatide leads to decrease in sleep apnea severity and body weight in SURMOUNT-OSA Study 2 participants using PAP therapy that can be explained by the primary and secondary endpoints of the trial.
Primary Endpoint
Tirzepatide reduced AHI by 30.4 events/hour vs 6.0 events/hour with placebo.
Secondary Endpoints
62.8% reduction in AHI with tirzepatide vs 6.4% with placebo. 51.5% of tirzepatide participants achieved disease resolution vs 13.6% with placebo. 74.3% of tirzepatide participants had ≥ 50%. AHI reduction vs 22.9% with placebo. 20.1% reduction in body weight with tirzepatide vs 2.3% with placebo [7].
Tirzepatide represents a promising breakthrough in the treatment of Obstructive Sleep Apnea (OSA), offering potential benefits that could reshape current management strategies. Tirzepatide has been reported to have a positive impact on OSA severity, weight loss whilst having positive effects on metabolic profiles. It could become a frontline therapy as applicable to patients suffering from obesity, CPAP which is usually perceived to be difficult for patients to follow.
Personalized Approach
The various ways by which tirzepatide impacts on the respiratory as well as metabolic mechanisms of OSA weighed the realistic approach towards tailored OSA treatment. That is why this individual patient-oriented approach can help to consider the specific needs of this or that patient and the potential causes of OSA.
CONCLUSION
Tirzepatide is a landmark strategy for the treatment of OSA in the great therapeutic arena. Clinical trials in particular, concern SURMOUNT-OSA and confirmed efficacy of Tirzepatide in reduction of severity of OSA and better performance compared to placebo in terms of AHI. By targeting both the metabolic abnormalities and the respiratory disturbances during sleep it is fairly effective than the conventional treatments. Further studies will show if it has sustained value in the long-term and will be certified as a key component in total OSA care and possibly even the overall wellbeing of its patients. Although many patients suffer from gastrointestinal side effects, tirzepatide shows a real potential in enhancing overall health of the patients with OSA which should be seen as a major breakthrough in the field, provided that further long- term effects should be investigated. Therefore, the broad- spectrum mechanism and clinical outcome of tirzepatide place it as the optimal intervention for people dealing with the challenges of OSAP and suggest it can recalibrate current standards in the field.
REFERENCES
- Slowik JM, Sankari A, Collen JF. Obstructive Sleep Apnea. In: StatPearls. Treasure Island (FL): StatPearls Publishing. 2024.
- De Block C, Bailey C, Wysham C, Hemmingway A, Allen SE, Peleshok J. Tirzepatide for the treatment of adults with type 2 diabetes: An endocrine perspective. Diabetes Obes Metab. 2023; 25: 3-17.
- U.S. Food and Drug Administration. FDA approves new medication for chronic weight management. 2023.
- Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022; 387: 205-216.
- Pamidi S, Aronsohn RS, Tasali E. Obstructive sleep apnea: role in the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab. 2010; 24: 703-715.
- Reutrakul S, Mokhlesi B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest. 2017; 152: 1070-1086.
- Malhotra A, Grunstein RR, Fietze I, Weaver TE, Redline S, Azarbarzin A, et al. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. N Engl J Med. 2024; 391:1193-1205.
- Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008; 60: 470-512.
- Holst JJ, Gasbjerg LS, Rosenkilde MM. The Role of Incretins on Insulin Function and Glucose Homeostasis. Endocrinology. 2021; 162: bqab065.
- Cao MT, Sternbach JM, Guilleminault C. Continuous positive airway pressure therapy in obstuctive sleep apnea: benefits and alternatives. Expert Rev Respir Med. 2017; 11: 259-272.
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008; 5: 173-178.
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021; 385: 503-515.
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008; 5: 136-143.
- Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol (1985). 2009; 106: 1538-1544.
- Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017; 390: 1664-1675.
- Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Investigation. 2005; 115: 3554-3563.
- Farzam K, Patel P. Tirzepatide. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
- Andraos J, Muhar H, Smith SR. Beyond glycemia: Comparing tirzepatide to GLP-1 analogues. Rev Endocr Metab Disord. 2023; 24: 1089-1101.
- Sardar MB, Nadeem ZA, Babar M. Tirzepatide: A novel cardiovascular protective agent in type 2 diabetes mellitus and obesity. Curr Probl Cardiol. 2024; 49: 102489.
- Pinto VL, Sharma S. Continuous Positive Airway Pressure. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2024.
- Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Benseñor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015; 70: 258- 264.
- Tietjens JR, Claman D, Kezirian EJ, De Marco T, Mirzayan A, Sadroonri B, et al. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J Am Coll Cardiol. 2022; 79: 293-307.
- Sardar MB, Nadeem ZA, Babar M. Tirzepatide: A novel cardiovascular protective agent in type 2 diabetes mellitus and obesity. Curr Probl Cardiol. 2024; 49: 102489.b