Effects of laser Therapy on Morselized Bone Allograft Incorporation Laser Therapy and Bone Grafts Incorporation
- 1. Department of Oral and Maxillofacial Surgery, Universidade do Planalto Catarinense (UNIPLAC) School of Dentistry, Lages, SC, Brazil.
- 2. Department of Statistic, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), and Universidade Federal do Rio Grande do Sul (UFRGS), School of Mathematics, Porto Alegre, RS, Brazil
- 3. Department of Microscopy and Microanalysis, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), School of Engineering, Porto Alegre, RS, Brazil.
- 4. Department of Veterinary Medicine, Universidade do Estado de Santa Catarina (UDESC), Lages, SC, Brazil.
- 5. Department of Surgery, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS) School of Dentistry, Porto Alegre, RS, Brazil.
- 6. Department of Oral and Maxillofacial Surgery, Hospital Cristo Redentor/Grupo Hospitalar Conceição (GHC), Porto Alegre, RS, Brazil
ABSTRACT
Objective: To assess the effect of low-level laser therapy (LLLT) on morselized bone allograft incorporation.
Material and Methods: Four rabbits were used as bone tissue donors. Morselized bone allografts and autografts were placed into the calvaria of 20 rabbits divided across four groups: two LLLT groups (allograft, autograft and blood clot) and two non-irradiated control groups (allograft, autograft and blood clot). Animals were euthanized at 35 days (n = 10) or 70 days (n = 10). LLLT consisted of spot laser irradiation (GaAlAs diode laser, λ = 830 nm, energy density 4 J/cm2 ) over four points on the skull, for a total of 16 J/cm2 per session and a total treatment dose of 128 J/cm2 .
Results: At 70 days, animals in the allograft + LLLT group had statistically significant differences in bone remodeling (p = 0.008) and vascularization (p = 0.032) as compared with the allograft control group. There were no statistically significant differences in bone incorporation in the allograft + LLLT and auto graft control groups.
Conclusion: Light microscopy and scanning electron microscopy analysis showed a qualitatively and quantitatively positive effect of LLLT on the speed of osteogenesis after morselized bone allograft placement.
KEYWORDS
• Transplantation
• Homologous
• Laser therapy
• Low-level
• Cryopreservation
CITATION
Paes JV, Valiati R, de Sá V. Paes FL, de Moraes JFD, et al. (2014) Effects of laser Therapy on Morselized Bone Allograft Incorporation Laser Therapy and Bone Grafts Incorporation. J Surg Transplant Sci 2(1): 1004.
INTRODUCTION
The experimental treatment of maxillofacial and craniofacial deformities with graft materials has undergone a series of improvements. The optimal bone graft material should be osteoinductive, so as to encourage osteogenesis, and osteoconductive, so as to provide a scaffold that establishes optimal conditions for the growth of blood vessels and cells with osteogenic potential. These requirements are met by autograft bone. However, donor site morbidity and limited volume have prompted the search for materials that can replace autogenous bone [1]. Autograft bone is the gold standard for reconstruction of severe alveolar defects, as it provides more predictable outcomes, poses no risk of disease transmission, and is fully histocompatible [2]. Allograft bone is essentially osteoconductive and provides a scaffold for cell migration, due to the presence of bone morphogenetic proteins, which are expressed after demineralization during human bone remodeling [3,4]. The extent of new bone formation between the graft and recipient bed is correlated with revascularization and healing time [5]. Incorporation of allogeneic bone at the graft–host interface occurs more slowly due to the greater initial inflammatory reaction and lower rate of revascularization [6]. Particulate bone and block graft are used in the reconstruction of maxillary defects and dental implant rehabilitation [6-8].
Bone graft particles obtained with a bone mill or bone scraper exhibit greater osteogenic potential than those obtained with a bone drill.9 GaAlAs laser irradiation has been shown to increase mechanical resistance at the bone–titanium implant interface. This effect is due to an increase in metabolic speed, accelerating the repair process [10,11]. Laser photobiomodulation is not detectable due to a great extent within 30 days of treatment, as the cell component is most prominent—and most susceptible to the effects of laser radiation—during the early stages of bone healing. Later on, the bone matrix is the main component of bone healing. Therefore, laser therapy is effective when carried out during the cell phase of bone repair, when the number of osteoblasts is on the rise [12,13]. Laser photobiomodulation therapy has a positive effect on early healing of bone defects [8,13]. When administered during the inflammatory period of the bone repair process, it increases normal cell activity (resorption and formation) [14]. A histologic study of the influence of GaAlAs laser radiation (λ = 830 nm) on the healing process of bone autografts in rats showed significant quantitative and qualitative increases in bone resorption and neoformation among animals who had undergone intraoperative laser irradiation of the wound bed, thus confirming the biomodulatory effects of low-level laser therapy [12].
MATERIAL AND METHODS
The study project was assessed and approved by the local Animal Experimentation Ethics Committee (CETEA – judgment no. 1.13.08). Twenty-four male New Zealand white rabbits (mean weight 4.0 kg, mean age 10 months) were used: four as tissue donors and 20 as experiment subjects, allocated into intervention and control allograft and auto graft groups (Table 1).
Table 1: Distribution of Study Groups.
Table 1: Distribution of Study Groups.
| Group | Treatment | Graft type | Incorporation period | N |
| Allograft Control | - | Morselized allograft | 35 days | 5 rabbits |
| Autograft Control | Morselized autograft | |||
| Clot Control | Blood clot | |||
| Allograft + Laser | Laser therapy | Morselized allograft | 35 days | 5 rabbits |
| Autograft + Laser | Morselized autograft | |||
| Clot + Laser | Blood clot | |||
| Allograft Control | - | Morselized allograft | 70 days | 5 rabbits |
| Autograft Control | Morselized autograft | |||
| Clot Control | Blood clot | |||
| Allograft + Laser | Laser therapy | Morselized allograft | 70 days | 5 rabbits |
| Autograft + Laser | Morselized autograft | |||
| Clot + Laser | Blood clot |
The frontal, parietal, and zygomatic areas of all animals were shaved in preparation for surgical access, as was the dorsal aspect of the ear (for marginal ear vein cannulation). During preanesthetic set-up, clinical parameters such as respiratory rate, heart rate, and capillary refill time were monitored and animals were premedicated with tiletamine/zolazepam 20 mg/kg and xylazine 3 mg/kg IM. Animals were then placed in the prone position (sternal recumbency) on an active warming pad and the marginal ear vein was cannulated with a 24G catheter for administration of normal saline solution (NaCl 0.9%) at a drip rate of 6 gtt/min. Maintenance of anesthesia was provided with isoflurane at 1.5 MAC (minimum alveolar concentration) in 100% oxygen, at 2 L/min via non-rebreather mask, using a universal anesthetic vaporizer. The frontoparietal region was further anesthetized with local infiltration of 0.5 mL plain lidocaine (2%). The surgical field was disinfected with povidone-iodine 1% and isolated with sterile drapes. An approximately 5 cm-long, full-thickness incision was made down to the periosteum overlying the sagittal suture and center of the frontal bone with a #15 blade and soft tissues were dissected with a periosteal elevator. In donor and recipient rabbits, ostectomy for removal of bone blocks from the calvaria was performed with a straight handpiece set to 800 rpm and an 8 mm trephine bur, under copious irrigation with saline solution. Block grafts were carefully elevated with a periosteal elevator without disturbing the integrity of the underlying dura and brain matter. Six bone blocks were obtained from the cranial vault of each donor rabbit, for a total of 24 block allografts. These allogeneic bone blocks were then rinsed with copious amounts of normal saline, separated from all soft tissues, and morselized in a bone mill (FAPESC 12388/2008-9) into macroparticles 1–2 mm in size. These particles were stored in sterile containers, flash-frozen at –70 °C, and kept in a deep freezer for 30 days before grafting. The four donor animals were euthanized immediately after the procedure with ketamine 50 mg/kg and 1 mg/kg diazepam, followed by a lethal injection of 600 mg potassium chloride into the marginal ear vein. After morselized bone allografts had been frozen for 30 days, recipient animals were anesthetized as described above and underwent graft placement. The incision and bone elevation procedures were as performed in the donor animals. Morselized bone was removed from its sterile packaging and thawed in normal saline at room temperature. The 8-mm trephine bur was used to remove a block autograft from the anterior-most region of the skull, to serve as a positive control of bone incorporation. This block was then morselized and grafted to the defect on the left, just as the morselized allograft was placed into the right-sided defect (packed with a periosteal elevator to ensure the greatest possible stability). After copious irrigation of the wound bed with normal saline, the incision was closed in a single plane with simple running sutures (5-0 nylon). Antimicrobial coverage was provided with enrofloxacin 5 mg/kg IM once daily. Postoperative analgesia consisted of meloxicam 0.1 mg/kg once daily, with rescue tramadol 2 mg/kg as needed in case of severe pain. Immediately after conclusion of the procedure, animals in group L (laser) underwent infrared laser irradiation with aluminum gallium arsenide diode laser (GaAlAs; wavelength 830 nm; Thera Lase®). The laser was applied to four sites overlying the graft areas (anterior, posterior, left lateral and right lateral aspects of the cranial vault), with the handpiece angled at 90° and 0.5 cm away from the skin, at an energy density of 4 J/cm2 , for a total dose of 16 J/cm2 per session. The total dose after eight sessions was therefore 128 J/cm2 . Irradiation was subsequently repeated every 48 hours over 14 days, for a total of eight sessions. Animals in group C (control) underwent sham irradiation, with the laser unit switched off, to simulate the stress of restraint. Five animals in each group (laser and control) were euthanized at 35 days (five weeks), and the remaining five in each group, at 70 days (10 weeks) post-graft implantation. Anesthesia was induced with tiletamine/zolazepam 20 mg/kg and xylazine 3 mg/kg IM, followed by a lethal injection of 300 mg potassium chloride into the marginal ear vein. After euthanasia, the surgical site was dissected and a 10-mm trephine bit was used to obtain a sample of bone containing the graft, blood clot (negative control) and host tissue margins. Specimens were placed into prepared and labeled bottles containing 10% buffered formalin solution, kept for 48 hours for fixation, decalcified in 5% aqueous nitric oxide, and cut lengthwise into 5-µm thick slices with a microtome knife. The resulting histological slides were stained with hematoxylin and eosin (H&E). For assessment of the effects of laser therapy on bone tissue, light microscopy was used for descriptive and semi quantitative histological examination. Images were obtained at x40 and x100 magnification using an Olympus® CX31RTSF optical microscope with DP 2 TWAIN camera and DP2-BSN Olympus Soft Imaging Solutions® software. For the parameters collagen fiber deposition, bone remodeling (physiological bone resorption by osteoclasts and new bone deposition by osteoblasts), filling of osteocyte lacunae, graft incorporation at the graft-to-host interface, inflammatory infiltration and vascularization, the following semi quantitative criteria were used: None – absent; Mild/Slight – present, < 25%, Moderate – present, 25% to 50%; and Marked – present, > 50%. Analysis was performed after calibration and each score was adjudicated three times to confirm the consistency of grading. The entirety of each slide was examined at x40 and x100 magnification. The other half of each sample was sent for scanning electron microscopy (SEM) at the Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS) Microscopy and Microanalysis Center (CEMM). The electron microscope used (Philips® XL30) has a resolution of up to 3.5 nm (in secondary electron mode), magnification ranges of approximately x25, x45, x250, x500 and x1000 and an accelerating voltage of 200V–30kV. Descriptive morphological analysis was conducted on the basis of images observed at the graft-to-host (recipient bed) interface, on longitudinal slices—at the center of the lateral walls between the recipient area and the graft area—of each sample, at the aforementioned magnifications. The Mann–Whitney U test was used for comparison of results between the control group and the experiment group for each of the variables of interest. The significance level was set at 5% and all analyses were carried out in SPSS® version 17 (Microsoft Corporation, USA). The variables collagen fiber deposition, bone remodeling, filling of osteocyte lacunae, graft incorporation, inflammatory infiltration and vascularization were operationalized by means of an interval scale of 0 to 3, with 0 (zero) being None – absent; 1 (one) being Mild/Slight – present, < 25%; 2 (two) being Moderate – present, 25% to 50%; and 3 (three) being Marked – present, > 50%.
RESULTS
Tables 2,3,4, and 5 show the results of statistical analysis of the aforementioned parameters of graft osseointegration (collagen fiber deposition, bone remodeling, filling of osteocyte lacunae, graft incorporation, inflammatory infiltration and vascularization).
Table 2: Statistical analysis (mann-whitney u) of the morselized bone allograft control vs. morselized bone autograft control groups at 35 and 70 days.
| Incorporation period Parameter | Treatment | 35 days N | 70 days Mean Rank | Mann-Whitney U | Incorporation period P value | Mean Rank | 35 days Mann-Whitney U | 70 days P value |
| Collagen fiber deposition | Allo C | 5 | 6.50 | 7.500 | 4.50 | |||
| Auto C | 5 | 4.50 | .310 | 6.50 | 7.500 | .310 | ||
| Total | 10 | |||||||
| Bone remodeling | Allo C | 5 | 5.00 | 10.000 | 4.00 | |||
| Auto C | 5 | 6.00 | .690 | 7.00 | 5.000 | .151 | ||
| Total | 10 | |||||||
| Filling of osteocyte lacunae | Allo C | 5 | 4.20 | 6.000 | 4.50 | |||
| Auto C | 5 | 6.80 | .222 | 6.50 | 7.500 | .310 | ||
| Total | 10 | |||||||
| Graft incorporation | Allo C | 5 | 3.40 | 2.000 | 0.32 | 6.00 | ||
| Auto C | 5 | 7.60 | 5.00 | 10.000 | .690 | |||
| Total | 10 | |||||||
| Inflammatory infiltrate | Allo C | 5 | 8.00 | .000 | .008 | 6.00 | ||
| Auto C | 5 | 3.00 | 5.00 | 10.000 | .690 | |||
| Total | 10 | |||||||
| Vascularization | Allo C | 5 | 5.50 | 12.500 | 1.000 | 4.00 | 5.000 | |
| Auto C | 5 | 5.50 | 7.00 | .151 | ||||
| Total | 10 |
Allo C: morselized bone allograft control group; Auto C: morselized bone autograft control group
Table 3: Statistical analysis (mann-whitney u) of the morselized bone allograft + laser vs. morselized bone autograft + laser groups at 35 and 70 days.
| Incorporation period | 35 days | 70 days | ||||||
| Parameter | Treatment | N | Mean Rank | Mann-Whitney U | P value | Mean Rank | Mann-Whitney U | P value |
| Collagen fiber deposition | Allo L | 5 | 6.40 | 8.000 | 5.00 | |||
| Auto L | 5 | 4.60 | .421 | 6.00 | 10.000 | .690 | ||
| Total | 10 | |||||||
| Bone remodeling | Allo L | 5 | 5.50 | 12.500 | 6.00 | |||
| Auto L | 5 | 5.50 | 1.000 | 5.00 | 10.000 | .690 | ||
| Total | 10 | |||||||
| Filling of osteocyte lacunae | Allo L | 5 | 3.00 | .000 | .008 | 5.00 | ||
| Auto L | 5 | 8.00 | 6.00 | 10.000 | .690 | |||
| Total | 10 | |||||||
| Graft incorporation | Allo L | 5 | 3.00 | .000 | .008 | 5.50 | ||
| Auto L | 5 | 8.00 | 5.50 | 12.500 | 1.000 | |||
| Total | 10 | |||||||
| Inflammatory infiltrate | Allo L | 5 | 8.00 | .000 | .008 | 6.00 | ||
| Auto L | 5 | 3.00 | 5.00 | 10.000 | .690 | |||
| Total | 10 | |||||||
| Vascularization | Allo L | 5 | 4.00 | 5.000 | 5.00 | |||
| Auto L | 5 | 7.00 | .151 | 6.00 | 10.000 | .690 | ||
| Total | 10 | |||||||
Allo L: morselized bone allograft + laser group; Auto L: morselized bone autograft + laser group
Table 4: Statistical analysis (mann-whitney u) of the morselized bone allograft control vs. morselized bone allograft + laser groups at 35 and 70 days.
| Incorporation period | 35 days | 70 days | ||||||
| Parameter | Treatment | N | Mean Rank | Mann-Whitney U | P value | Mean Rank | Mann-Whitney U | P value |
| Collagen fiber deposition | Allo C | 5 | 5.00 | 5.50 | ||||
| Allo L | 5 | 6.00 | 10.000 | .690 | 5.50 | 12.500 | 1.00 | |
| Total | 10 | |||||||
| Bone remodeling | Allo C | 5 | 4.50 | 3.00 | ||||
| Allo L | 5 | 6.50 | 7.500 | .310 | 8.00 | 0.000 | 0.008 | |
| Total | 10 | |||||||
| Filling of osteocyte lacunae | Allo C | 5 | 5.50 | 4.50 | ||||
| Allo L | 5 | 5.50 | 12.500 | 1.000 | 6.50 | 7.500 | 0.310 | |
| Total | 10 | |||||||
| Graft incorporation | Allo C | 5 | 5.00 | 5.50 | ||||
| Allo L | 5 | 6.00 | 10.000 | .690 | 5.50 | 12.500 | 1.000 | |
| Total | 10 | |||||||
| Inflammatory infiltrate | Allo C | 5 | 7.40 | 5.50 | ||||
| Allo L | 5 | 3.60 | 3.000 | .056 | 5.50 | 12.500 | 1.000 | |
| Total | 10 | |||||||
| Vascularization | Allo C | 5 | 5.50 | 3.50 | ||||
| Allo L | 5 | 5.50 | 12.500 | 1.000 | 7.50 | 2.500 | 0.032 | |
| Total | 10 | |||||||
Allo C: morselized bone allograft control group; Allo L: morselized bone allograft + laser group
Table 5: Statistical analysis (mann-whitney u) of the morselized bone autograft control vs. morselized bone allograft + laser groups at 35 and 70 days.
| Incorporation period | 35 days | 70 days | ||||||
| Parameter | Treatment | N | Mean Rank | Mann-Whitney U | P value | Mean Rank | Mann-Whitney U | P value |
| Collagen fiber deposition | Auto C | 5 | 6.80 | 6.000 | 4.50 | |||
| Allo L | 5 | 4.20 | .222 | 6.50 | 7.500 | .310 | ||
| Total | 10 | |||||||
| Bone remodeling | Auto C | 5 | 6.00 | 10.000 | .690 | 6.50 | ||
| Allo L | 5 | 5.00 | 4.50 | 7.500 | .310 | |||
| Total | 10 | |||||||
| Filling of osteocyte lacunae | Auto C | 5 | 4.20 | 6.000 | 5.50 | |||
| Allo L | 5 | 6.80 | .222 | 5.50 | 12.500 | 1.000 | ||
| Total | 10 | |||||||
| Graft incorporation | Auto C | 5 | 3.50 | 2.500 | 0.32 | 6.00 | ||
| Allo L | 5 | 7.50 | 5.00 | 10.000 | .690 | |||
| Total | 10 | |||||||
| Inflammatory infiltrate | Auto C | 5 | 8.00 | .000 | .008 | 6.00 | ||
| Allo L | 5 | 3.00 | 5.00 | 10.000 | .690 | |||
| Total | 10 | |||||||
| Vascularization | Auto C | 5 | 5.50 | 12.500 | 6.00 | |||
| Allo L | 5 | 5.50 | 1.000 | 5.00 | 10.000 | .690 | ||
| Total | 10 | |||||||
Allo L: morselized bone allograft + laser group; Auto C: morselized bone autograft control group
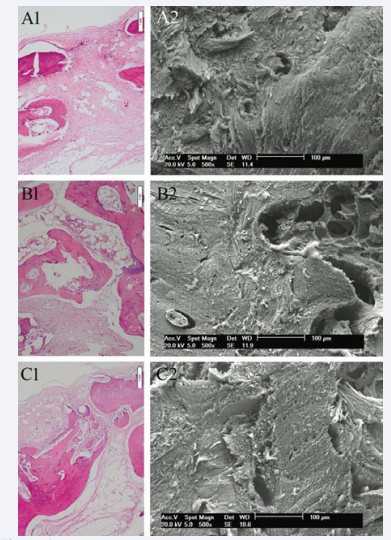
Both light microscopy and SEM showed graft incorporation at the graft-to-host interface in all experimental specimens. At the graft edges, there was close contact with the recipient area, with filling of osteocyte lacunae, and medullary degeneration and necrosis in the medullary spaces of the graft-to-host interface, with neovascularization, collagen fiber deposition and bone remodeling. Remodeling was less marked toward the graft center. Analysis provided evidence of the osteoconductive properties of morselized allograft bone stored by a deep-freezing process, which preserved the structural integrity of the graft material. The bone matrix served as a scaffold for host-to-graft migration of cells and blood vessels. At 35 days, there was moderate collagen fiber deposition in the allograft + laser and allograft control groups and mild-to-moderate collagen fiber deposition throughout the medullary space in the autograft control group. Mild-to-moderate bone remodeling was seen in the allograft control group, versus moderate remodeling in the allograft + laser and auto graft control groups. Most osteocyte lacunae were filled in all laser and control group specimens. A mononuclear cell infiltrate—mild in the irradiated groups and moderate to marked in the allograft and auto graft control groups—was visible in the medullary spaces. At 70 days (Figure 1), presence of collagen fibers in the medullary spaces was slight in the allograft + laser and allograft control groups and slight to moderate in the auto graft control group.
Figure 1: At 70 days, (A1) Morselized allograft control group (H&E, original magnification x100); (A2) Morselized allograft control group (SEM, original magnification x500); (B1) Morselized allograft + laser group (H&E, original magnification x100); (B2) Morselized allograft + laser group (SEM, original magnification x500); (C1) Morselized autograft control group (H&E, original magnification x100); (C2) Morselized autograft control group (SEM, original magnification x500).
There was marked bone remodeling in the allograft + laser group, versus moderate remodeling in the allograft control group and moderate to marked remodeling in the auto graft control group. In the laser and auto graft control groups, most lacunae were markedly filled with osteocytes; in the allograft control group, filling was moderate to mark. Vascularization was marked in the allograft + laser and auto graft control groups, and moderate in the allograft control group. At 35 and 70 days, in the blood clot control and blood clot + laser groups, bone remodeling was qualitatively and quantitatively inferior as compared with the graft groups. At 35 days, collagen fiber deposition and bone remodeling were slight to moderate in the laser group and slight in the control group. Filling of osteocyte lacunae in the laser and control groups was slight or absent. Inflammatory infiltrate and vascularization were mild/slight to moderate in both groups. At 70 days, filling of osteocyte lacunae and vascularization were moderate to mark. Collagen fiber deposition, bone remodeling, and inflammatory infiltration were slightly better than in the 35-day group.
DISCUSSION
Whereas allograft bone is osteoconductive and weakly osteoinductive, autografts are osteogenic, osteoinductive and osteoconductive. Auto grafts do not pose a risk of graft rejection or disease transmission, but there is retention of viable osteoblasts. Allograft is more widely available through bone and tissue banks, can be obtained in custom shapes, and do not require an additional surgical procedure. Auto grafts feature disadvantages such as morbidity and limited availability, whereas allograft are immunogenic, not osteogenic [15-17]. Allograft bone has been used at least since the 1970s for correction of maxillofacial deformities, with outcomes quite similar to those of autogenous grafts, but with slower resorption and incorporation. [18] In this study, we used a rabbit animal model, as in the previous work of Khadra et al. [11] and Campanha et al., [18] due to ease of handling, availability, and need for a larger bone surface area for assessment of the effects of laser therapy on morselized (particulate) bone grafts. Autograft collection and morselized allograft implantation required construction of three 8-mm defects in the same animal. The cranial vault was used due to its larger surface as compared with the femur or tibia, which are used in most studies of laser therapy for biomodulation of bone regeneration in small (1 to 5 mm) defects [1,12,13]. Particulate bone grafts are more quickly revascularized, release greater amounts of differentiation and growth factors at the early stages of regeneration, and exhibit more intense osteoclast activity— resulting in greater resorption—as compared with block graft [19]. Graft volume and its placement onto the recipient bed are believed to have a major impact on the extent and quality of bone incorporation, as large volumes take longer to incorporate or are never fully incorporated [5]. In view of the systemic effects of laser, we allocated animals to irradiation and no-irradiation (control) groups, as in the vast majority of studies. As the systemic effects of laser therapy can never be ruled out, some studies using a contralateral animal-as-own-control design may have failed to reveal significant effects of irradiation [10-12,14,17].
At 70 days, animals in the laser-irradiated bone allograft groups exhibited significant quantitative and qualitative differences in the parameters bone remodeling and vascularization when compared with the non-irradiated allograft control groups. These findings corroborate those of authors who have reported greater bone remodeling and vascularization in animals exposed to laser therapy [5,13,14,20]. In the present study, there were statistically significant differences in the groups assessed at 35 days: the autograft control group exhibited greater graft incorporation and less inflammatory infiltration as compared with the allograft + laser group. This is because a 35-day period in rabbits is equivalent to 3–4 months in humans, a point in time at which the bone incorporation process is still incomplete. Due to the superior cell characteristics of autologous bone, there was a significant difference between the allograft and autograft groups. Conversely, at 70 days, morselized bone allograft was an effective substitute for autograft bone, as the allograft groups who underwent laser therapy exhibited no significant differences from the autograft control groups, which proves that LLLT speeds the allograft incorporation process. From a quantitative standpoint, animals in the allograft + laser group exhibited better bone remodeling and vascularization at 70 days than the non-irradiated control group.
Regarding the number of LLLT sessions, published studies on the theme have reported between one and 14 irradiations. In the present study, we carried out eight sessions: one in the immediate postoperative period and seven more every 48 hours thereafter, as in Weber et al. [12] Other studies that have used seven sessions of LLLT to positive effect include Pinheiro et al. [13] and Campanha et al. [18] Multiple applications are more effective than a single dose in terms of inducing bone formation and fibroblast growth [13]. Regarding laser wavelength and diode type, the available literature is quite diverse. Some researchers have used lasers with wavelengths in the 680–690 nm range, without reporting the diode type [10,17,20]. The majority of published studies have used aluminium gallium arsenide (GaAlAs) laser with a wavelength of 830 nm [11,12,18] or GaAlAs with λ = 670 nm[13] or λ = 660 nm, [14] as well as HeNe laser with λ = 632.8nm [18]. In this study, we used an 830- nm GaAlAs laser, as in most of the existing literature, due to the size of the defect (8 mm) into which morselized bone grafts were placed, occupying a significant volume into which infrared laser radiation could achieve deep penetration. Laser can penetrate up to 2–3 cm [11], especially in the subcutaneous tissues [12].
Optical microscopy and scanning electron microscopy showed that allograft bone processed by deep-freezing plus LLLT is suitable as an alternative for the treatment of bone defects. Use of the deep-freezing method for processing of bone grafts preserves the structural and osteoconductive characteristics of bone tissue [8].
All groups exhibited incorporation of their bone grafts. The characteristics of union between allogeneic bone and the recipient area suggest host acceptance. The agglomeration of morselized bone graft particles associated with tight packing and complete filling of the bone defect encouraged good adaptation of graft material to skull defects and intermeshing of graft morsels and bone cells, as well as vascularization of the recipient bed [12]. At the alveolar ridges and maxillary sinuses, the mean expected increase is 4 to 5 mm in thickness and 1 to 2 mm in height[3], which explains the fact that biopsies obtained at the time of implant placement in the maxilla or mandible reveal neoformed bone tissue and no residual material[21,22]. The osseointegration reported in some cases is also a strong indicator of bone integrity. Were that not the case, implant loss rates would be higher than reported [3].
CONCLUSION
In conclusion, this study revealed a qualitative and quantitative positive effect of LLLT on the speed of osteogenesis in morselized bone grafts across all groups. Comparison between morselized allografts exposed to laser therapy and non-irradiated morselized autografts showed no statistically significant differences at 70 days, showing that LLLT sped incorporation of the allograft to such an extent that it was equivalent to autogenous bone.
ACKNOWLEDGEMENTS
?The authors would like to thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brazil) and FAPESC (Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina).
? This study should be attributed to the Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS). Address: Av. Ipiranga, 6681, Prédio 6, Odontologia, Campus Central, CEP: 90619-900, Porto Alegre, RS, Brazil. Phone/Fax: +55 51 33203539
Funding acknowledgement
CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) – Brazil.