The Role of Tranexamic Acid (TXA) In Spine Surgeries: A Systematic Review
- 1. Department of Surgery, Iraq
- 2. Department of Neuroscience, Saint Georges Hospital, London
CITATION
Almahfoodh AB, Lui DF, Hamdan TA (2021) The Role of Tranexamic Acid (TXA) In Spine Surgeries: A Systematic Review. J Surg Transplant Sci 8(1): 1083.
INTRODUCTION
Tranexamic Acid (TXA)
Tranexamic acid (TXA) was first made in 1962 by Japanese researchers Shosuke and Utako Okamoto. [1]
Pharmacokinetics
Tranexamic acid is a synthetic analog of the amino acid lysine. It serves as an antifibrinolytic by reversibly binding four to five lysine receptor sites on plasminogen. This reduces conversion of plasminogen to plasmin, preventing fibrin degradation and preserving the framework of fibrin’s matrix structure. [2]
It can be administered through several routes ,orally, topically, or intravenously, and has a 100% bioavailability [8]
Intravenous administration results in peak levels within minutes. The average half-life (time needed for half the active drug to be eliminated from the body) is two hours following intravenous administration and up to 12 hours following oral administration. [7]
95% of Tranexamic acid is excreted unchanged in the urine and only small amount is metabolized by the Liver.[2]
Dosing
Dosing of TXA differs according to the Indication, but regarding it’s usage in Spine Surgeries Previous studies have demonstrated the clinically effective intravenous dose to be 10-15 mg/kg of body weight, with higher dosages providing diminishing benefits.[9]
A topical TXA irrigation is typically poured into the surgical field before wound closure, with dosing protocols ranging from 10 mg/kg (i.e., around 1 g) to 3 g of tTXA in saline solution. [10]
Indications
Tranexamic acid is frequently used following major trauma [3]. Tranexamic acid is used to prevent and treat blood loss in a variety of situations, such as dental procedures for hemophiliacs, heavy menstrual bleeding, and surgeries with high risk of blood loss. [4-5] it is also used in orthopedic surgery to reduce blood loss, to the extent of reducing or altogether abolishing the need for perioperative blood collection. It is of proven value in clearing the field of surgery and reducing blood loss when given before or after surgery. Drain and number of transfusions are reduced. [6]
Contraindications
• Allergy to tranexamic acid.
• History of seizures.
• History of venous or arterial thromboembolism or active thromboembolic disease.
• Severe kidney impairment due to accumulation of the medication, so dose adjustment is required in mild or moderate kidney impairment. [11]
Adverse Effects
Side effects are rare [12]. Some include changes in color vision, blood clots, and allergic reactions[12].
Blood clots may include venous thromboembolism (deep vein thrombosis and pulmonary embolism), anaphylaxis [2]. These rare side effects were reported in post marketing experience and frequencies cannot be determined [2]. Despite the mode of action, large studies of the use of tranexamic acid have not shown an increase in the risk of venous or arterial thrombosis [13].
SPINE SURGERY
Massive blood loss occurs frequently and remains a challenge in spinal surgery [14]. Significant intra- and postoperative hemorrhage negatively affects patient outcomes by increasing coagulopathy, postoperative hematoma, and anemia [15]. The need for allogenic blood transfusions can lead to potential transfusion reactions and infections, in addition to increasing long-term mortality rates. [15]There is an economic disadvantage associated with iatrogenic major blood loss relating to the direct costs of the blood products and intraoperative blood salvage technology and indirect costs of prolonged patient hospitalization and complication management [16].
There are actually increasing numbers of clinical trials and retrospective studies investigating the role of TXA in spine surgeries, using different doses, different methods of administration and on different types Surgeries , THIS Meta -Analysis is directed towards finding the missing points regarding those Studies , and hopefully will guide us towards a more targeted research regarding this topic in the future to help overcome the limitations of TXA use in Spine Surgeries perse and orthopedic practice in general.
METHODS
The systematic review was written by in guidance of an adapted version of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Search Strategy
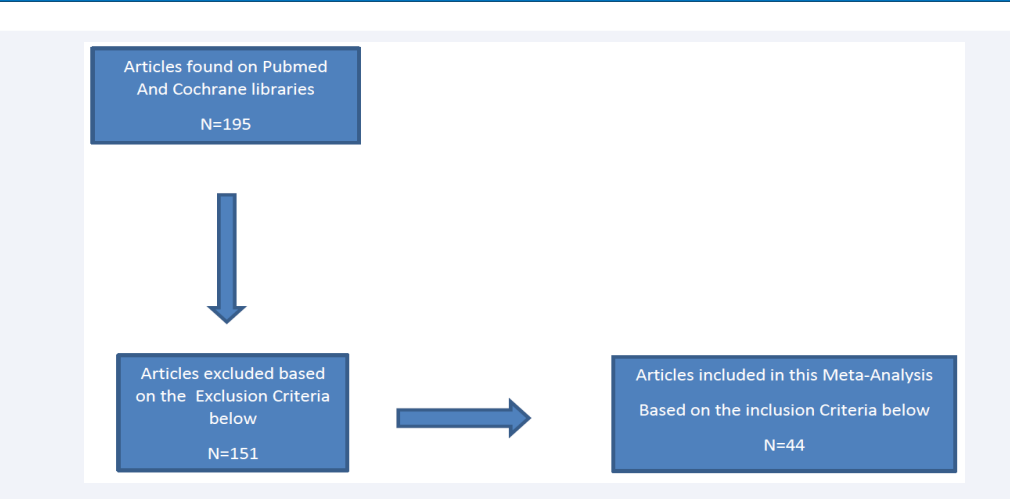
An electronic database search was conducted on PubMed and Cochrane electronic databases. As seen in (Figure 1),
Figure 1: Example of the searching on PubM.
Flow chart 1: Study Selection
the search terms “The Role of Tranexamic Acid in Spine Surgeries” were crucial in our database search in enabling us to find articles relevant to our inclusion criteria (which is detailed below)
Inclusion Criteria:
1-The population of the Study Should be patients undergoing Spine surgeries regardless the Type.
2-Only Tranexamic acid must be used and no other antifibrinolytic agent regardless the method of administration.
3-Studies designed to find the effect of TXA on intra and/or postoperative Bleeding and /or the need for postoperative blood Transfusion.
xclusion Criteria :
1-Meta analysis Designed Studies.
2-Studies and trials in which other Antifibrinolytic agents were used.
3-Studies that are designed to investigate the Role Of tranexamic Acid in orthopedic practice in General.
4- Studies that were designed to compare the Difference between Different methods of administration and Different doses.
5-Studies that are designed to investigate the effect of TXA on wound complications.
6-Duplicated titles.
7-Studies not related to spinal Surgeries.
Table 1: Studies Investigated.
|
No |
Authors |
Article title |
Method of Administration |
Type of spine surgery |
Result &conclusion |
|
1 |
Louanne M Carabini , Natalie C Moreland , Ryan J Vea- ley , John F Bebawy , Tyler R Koski , Antoun Koht , Dhanesh K Gupta , Michael J |
A Randomized Controlled Trial of Low-Dose Tranexamic Acid versus Placebo to Reduce Red Blood Cell Transfusion During Complex Multilevel Spine Fu- sion Surgery(17). |
IV TXA (10 mg/kg load- ing dose, then 1 mg/kg/ hr throughout surgery). |
Complex Multi- level Spine Fu- sion Surgery. |
Our results support the use of low-dose tranexamic acid during complex multilevel spine fusion surgery to de- crease total red blood cell transfusion. |
|
2 |
Weera Sudprasert, Terdpong Tanaviriyachai , Kongtush Choovongkomol , Sarut Jong- kittanakul , Urawit Piyaprom- dee. |
A Randomized Controlled Trial of Topical Application of Tran- examic Acid in Patients with Thoracolumbar Spine Trauma Undergoing Long-Segment Instrumented Posterior Spinal Fusion(18). |
solution containing 1 g of TXA (20 mL) was applied to the site of surgery via a drain tube after the spinal fascia was closed, and then the drain was clamped for 2 hours. |
Long-Segment Instrume-nted Posterior Spinal Fusion |
The use of topically admin- istered 1 g TXA in thoracic and lumbar spinal trauma cases effectively decreased postoperative transfusion requirements and mini- mized postoperative blood loss, as determined by the total drainage volume. |
|
3 |
Cheng-Cheng Yu , Wen-Jie Gao, Jun-Song Yang, Hua Gu, Ming Zhu Md, Kai Sun, Ding-Jun Hao. |
Can tranexamic acid reduce blood loss in cervical laminec- tomy with lateral mass screw fixation and bone grafting: a retrospective observational study(19). |
IV TXA. |
cervical lamin- ectomy with lat- eral mass screw fixation and bone grafting. |
Total blood loss in the TXA group was significantly lower than that of the con- trol group. |
|
4 |
Yufu ou, , Jianxun Wei , Rongzhu Li , Bin Liang , Dezan Qiu , Minke Wei , Xiaoping Mu , Zhuhai Li. |
Clinical Research of Combined Intravenous Administration and Topical Application of Tranexamic Acid to a Surgical Wound During Posterior Lum- bar Fusion(20). |
IV And topical TXA. |
double-segment posterior lumbar decompression and fusion sur- gery. |
Combined intravenous and topical administration of TXA seems to be effective and safe in reducing al- logenic blood transfusion and blood loss in double- segment posterior lumbar decompression and fusion surgery. |
|
5 |
Jeff Ehresman, Zach Pen- nington , Andrew Schill- ing , Ravi Medikonda , Sakibul Huq , Kevin R Merkel , A Karim Ahmed , Ethan Cottrill , Daniel Lubelski , Erick M West- broek , Salia Farrokh , Steven |
Cost-benefit analysis of tranexamic acid and blood transfusion in elective lumbar spine surgery for degenerative pathologies (21). |
IV TXA. |
elective lumbar spine surgery for degenerati-ve pathologie-s. |
TXA use was associated with decreased intraopera- tive blood loss and signifi- cant reductions in total he- mostasis costs for patients undergoing surgery on more than 4 levels. |
|
6 |
Xiji Wang , Ruize Yang , Hong- |
Different Effects of Intrave- nous, Topical, and Combined Application of Tranexamic Acid on Patients with Thoracolum- bar Fracture (22). |
IV , Topical and Combined TXA. |
thoracolumbar fracture fixation with percuta- neous pedicle screw. |
Preoperative intravenous drip of TXA can remarkably reduce intraoperative HBL and IBL in patients with thoracolumbar fracture fixed with percutaneous pedicle screw. |
|
7 |
Evan Larson , Tyler Evans, Jake Long, Emmett Gannon, Eliza- beth Lyden, Chris Cornett. |
Does Prophylactic Administra- tion of TXA Reduce Mean Oper- ative Time and Postoperative Blood Loss in Posterior Ap- proach Lumbar Spinal Fusion Surgery Performed for Degen- erative Spinal Disease?(23). |
IV TXA. |
Lumbar Spinal Fusion Surgery Performed for Degenerative Spinal Disease. |
In the present study, peri- operative TXA administra- tion was associated with re- duced postoperative drain output and surgical time. |
|
8 |
|
Does Tranexamic Acid Reduce Perioperative Bleeding in Short Segment Pedicle Screw Fixation in Thoracolumbar Spine Fractures?(24). |
10 mg/kg of TXA 30 min- utes intravenously before skin incision and 3 hours post-operative and oral medication for three days. |
Short Segment Pedicle Screw Fixation in Thoracolumbar Spine Fractures. |
Administration of TXA be- fore surgery significantly reduces perioperative bleeding in patients under- going short segment pedicle screw fixation for thoraco- lumbar spine fractures. |
|
9 |
Signe Elmose, Mikkel Ø Andersen, Else Bay An- dresen, Leah Yacat Carreon. |
Double-blind, randomized con- trolled trial of tranexamic acid in minor lumbar spine surgery: no effect on operative time, intraoperative blood loss, or complications(25). |
IV TXA (10 mg/kg). |
minor lumbar decompressive surgery. |
Tranexamic acid did not have a statistically sig- nificant effect on operative time, intraoperative blood loss, or complications. |
|
10 |
Ho Yong Choi , Seung-Jae Hyun , Ki-Jeong Kim , Tae-Ahn Jahng , Hyun-Jib Kim. |
Effectiveness and Safety of Tranexamic Acid in Spinal De- formity Surgery(26). |
IV TXA. |
spinal deformity surgery. |
TXA use can effectively reduce the amount of intra- operative bleeding and transfusion requirements in spinal deformity surgery. |
|
11 |
Sherif Elwatidy, Zain Jam- joom, Essam Elgamal, Amro Zakaria, Ahmed Turkista- ni, Abdelazeem El-Dawlatly. |
Efficacy and safety of prophy- lactic large dose of tranexamic acid in spine surgery: a pro- spective, randomized, double- blind, placebo-controlled study(27). |
Patients received either TA or placebo as a loading dose of 2 g (for adults) or 30 mg/kg (for children), followed immediately by continuous infusion of 100 mg/h (for adults) or 1 mg/ kg/h (for children) during surgery and for 5 hours after the operation. |
Eighteen patients had multilevel anterior cervi- cal discectomies with or without internal fixation, 22 patients had decompressive surgery (12 lami- nectomies and 10 intersegmental decompressions) for multiseg- ment spinal ste- nosis, 15 patients had laminectomy with posterior spinal fixation, and remaining 9 patients had laminectomy and excision of spinal tumor. |
Prophylactic use of large doses of TA provides an effective, safe, and cheap method for reducing blood loss during and after spinal operations. |
|
12 |
Zhinan Ren , Shugang Li, Lin Sheng, Qianyu Zhuang, Zheng Li, Derong Xu, Xin |
Efficacy and Safety of Topical Use of Tranexamic Acid in Re- ducing Blood Loss During Pri- mary Lumbar Spinal Surgery: A Retrospective Case Control Study(28). |
Wound surface was soaked with TXA (1 g in 100 mL saline solution) for 5 minutes before wound closure. |
Posterior lumbar spinal fusion surgery. |
Topical TXA can signifi- cantly reduce postopera- tive blood loss, accelerate removal of drainage tube, shorten the duration of hospital stay, while not increasing the complication incidence in patients un- dergoing posterior lumbar spinal fusion surgery. |
|
13 |
Majid R Farrokhi , Asef P Kazemi, Hamid R Eftekhar- ian, Kamal Akbari. |
Efficacy of prophylactic low dose of tranexamic acid in spinal fixation surgery: a rand- omized clinical trial (29). |
TXA (10 mg/kg) at the initiation of induction of anesthesia during 10 min followed by intravenous infusion of 1 mg/kg/h. |
Spinal fixation surgeries. |
The administration of a pro- phylactic low dose of TXA did not have a significant effect in the management of intraoperative blood loss and transfusion require- ments in patients undergo- ing spinal fixation surgery. |
|
14 |
|
Efficacy of tranexamic acid in reducing blood loss in poste- rior lumbar spine surgery for degenerative spinal stenosis with instability: a retrospective case control study(30). |
patients received 1 g tran- examic acid intravenous, preoperative and six hours and twelve hours postop- erative. |
posterior lumbar spine surgery for degenerative spi- nal stenosis with instability. |
This study suggests a less blood loss when admin- istering tranexamic acid in posterior lumbar spine surgery as demonstrated by the higher postoperative hemoglobin concentration and the less blood loss. |
|
|
|
|
|
|
|
|
15 |
|
High-Dose Tranexamic Acid Reduces Blood Loss in Exten- sive Spine Surgery(31). |
The tranexamic acid group received an intravenous loading dose of 100 mg/ kg over 20 minutes before skin incision. |
corrective sur- gery for spinal deformities, in- cluding posterior vertebral column resections |
Blood loss and blood trans- fusion requirements were all significantly lower in the tranexamic acid group |
|
16 |
|
High-dose tranexamic acid reduces intraoperative and postoperative blood loss in posterior lumbar interbody fusion(32). |
patients received 2000 mg of intravenous TXA 15 minutes before the skin in- cision was performed and received the same dose again 16 hours after the surgery. |
posterior lumbar interbody fusion. |
High-dose TXA significantly reduced both intra- and postoperative blood loss without causing any com- plications during or after single-level PLIF. |
|
17 |
M J Colomina , M Koo , M Baso- ra , J Pizones , L Mora , J Bagó |
Intraoperative tranexamic acid use in major spine surgery in adults: a multicenter, rand- omized, placebo-controlled trial(33). |
IV TXA. |
Posterior instru- mented spine surger. |
TXA did not significantly reduce transfusion require- ments, but significantly re- duced perioperative blood loss in adults undergoing major spinal surgery. |
|
18 |
Moslem Shakeri , Firooz Saleh- pour , Ghaffar Shokouhi , Kam- kar Aeinfar , Javad Aghaza- deh , Farhad Mirzaei , Seyed Ahmad Naseri Alavi. |
Minimal Dose of Tranexamic Acid Is Effective in Reducing Blood Loss in Complex Spine Surgeries: A Randomized Double-Blind Placebo Control- led Study(34). |
preoperative single doses of intravenous TXA (15 mg/kg). |
laminectomy and posterior spinal fusion. |
The findings of this study suggest that TXA can reduce both intraoperative and immediate postoperative blood loss, decrease the need for packed cell trans- fusion, and reduce the dura- tion of hospitalization after complex spinal surgeries. |
|
19 |
Jingming Xie , Lawrence G Lenke , Tao Li , Yongyu Si , Zhi Zhao , Yingsong Wang , Ying Zhang , Jie Xiao. |
Preliminary investigation of high-dose tranexamic acid for controlling intraoperative blood loss in patients under- going spine correction sur- gery(35). |
TXA group received an intravenous loading dose of 100 mg/Kg over a 20-minute period, fol- lowed by a maintenance infusion of 10 mg/Kg/h until skin closure was completed. |
spinal deformi- ties undergoing spinal corrective surgeries. |
high doses of TXA have been shown to effectively control blood loss and reduce the transfusion re- quirement. |
|
20 |
Dong , Huan Ming Wang , Fei Hu , Qiang Shao , Xu Chen , Lang Chen. |
Safety and efficacy of tran- examic acid in spinal canal tumors: a retrospective cohort study(36). |
The TXA dose regimen in group A comprised a loading dose of 10 mg/ kg 30 minutes before the operation, followed by a maintenance dose of 1 mg/kg per hour during the operation. |
Surgeries for spinal canal tu- mors. |
TXA used during spinal tumor surgery can reduce the amount of intraopera- tive blood loss and postop- erative drainage without increasing the risk of deep vein thrombosis and related complications. |
|
21 |
Roberto J Perez-Roman , Ju- lian G Lugo-Pico , Joshua |
Short-term safety of tran- examic acid use in posterior cervical decompression and fusion surgery (37). |
IV TXA. |
posterior cervical decompression and fusion sur- gery. |
Post-operative blood was significantly reduced (453 ml vs 701 ml; p = 0.03) in the group that received TXA. There was also a sig- nificant reduction in dura- tion of surgery associated with TXA use. |
|
22 |
Zhuang Zhang , Lin-Nan Wang , Xi Yang , Li-Min Liu Peng Xiu , Zhong-Jie Song. |
The effect of multiple-dose oral versus intravenous tranexamic acid in reducing postoperative blood loss and transfusion rate after adolescent scoliosis sur- gery: a randomized controlled trial(38). |
Patients received intrave- nous TXA 50 mg/kg load- ing dose and 10 mg/kg/h maintenance dose during surgery. Group A received 1 g oral TXA at 4 hours, 10 hours, and 16 hours postoperatively; group B received 0.5 g intravenous TXA at 6 hours, 12 hours, and 18 hours postopera- tively; group C received placebo. |
adolescent scol- iosis surgery. |
A multiple-dose regimen of TXA, either by oral or intravenous application, could be a safe and effective means of controlling PBL and decreasing the postop- erative transfusion rate in patients with AIS who un- derwent scoliosis surgery. In addition, it could inhibit postoperative inflammatory response. |
|
23 |
Alexander F Haddad , Chris- topher P Ames , Michael Safa- ee , Vedat Deviren , Darryl Lau. |
The Effect of Systemic Tran- examic Acid on Hypercoagu- lable Complications and Peri- operative Outcomes Following Three-Column Osteotomy for Adult Spinal Deformity(39). |
IV TXA. |
Three-Column Osteotomy for Adult. |
Systemic TXA use during 3CO for ASD surgery was not associated with de- creased blood loss. TXA pa- tients had shorter operative times, but this was driven mainly by surgeon experi- ence on multivariate analy- sis. Routine use of TXA is safe and does not increase the incidence of hyperco- agulable complications even at high doses. |
|
24 |
Marios G Lykissas , Alvin H Crawford , Gilbert Chan , Lori A Aronson , Mohammed J Al- Sayyad. |
The effect of tranexamic acid in blood loss and transfusion vol- ume in adolescent idiopathic scoliosis surgery: a single- surgeon experience(40). |
IV TXA. |
posterior spinal fusions per- formed for ado- lescent idiopathic scoliosis. |
TXA significantly decreased intraoperative blood loss in posterior spinal fusions performed for adolescent idiopathic scoliosis. |
|
25 |
Hong Sun , Luoyi Deng , Jin |
The Efficacy and Safety of Pro- phylactic Intravenous Tran- examic Acid on Perioperative Blood Loss in Patients Treated with Posterior Lumbar Inter- body Fusion(41). |
IV TXA. |
Posterior Lum- bar Interbody Fusion. |
A prophylactic intravenous administration of TXA 30 minutes before skin inci- sion effectively reduces the perioperative blood loss, duration of tube drainage, and hospitalization time, and it does not increase the risk of complications. However, TXA may not be able to decrease the rate of blood transfusion. |
|
26 |
Peng Xue , Junsong Yang , Xi- aozhou Xu , Tuanjiang Liu , Yansheng Huang , Feng Qiao , Xiaoqiang Huang. |
The efficacy and safety of tranexamic acid in reducing perioperative blood loss in pa- tients with multilevel thoracic spinal stenosis: A retrospective observational study(42). |
Intravenous infusion of 15 mg/kg 15 min prior to surgery. |
treatment of multilevel tho- racic spinal canal stenosis using trabeculectomy with posterior laminectomy and posterolateral bone graft fusion. |
TXA can reduce the amount of blood transfused and the need for blood transfusion and can shorten the extuba- tion time and the length of postoperative hospital stay without increasing the incidence of postoperative coagulation dysfunction or postoperative DVT. |
|
27 |
Mereze Visagie , Caroline X Qin , Brian C Cho , Kevin R Merkel , Tymoteusz J Kajstu- ra , Raj M Amin , Taylor E Pur- vis , Khaled M Kebaish , Steven M Frank. |
The impact of patient blood management on blood utiliza- tion and clinical outcomes in complex spine surgery(43). |
IV TXA. |
Complex spine surgery. |
In complex spine surgery, a multifaceted PBM program that includes TXA can be advantageous by reducing transfusion requirements without changing clinical outcomes. |
|
28 |
Jin-Qian Liang , Tian-Hua Rong , Hong-Zhe Liu , Ming- Sheng Tan , Hong Zhao , Xiang- Yang Liu , Lei Chang. |
Topical Injection of Tranexam- ic Acid via a Drain Plus Drain- Clamping to Reduce Blood Loss in Degenerative Lumbar Scoliosis Surgery(44). |
Topical Injection of Tran- examic Acid via a Drain Plus Drain-Clamping. |
Degenerative Lumbar Scoliosis Surgery. |
Topical injection of TXA retrogradely via a drain at the end of a degenerative lumbar scoliosis operation and clamping the drain for an hour can effectively decrease the postoperative blood loss and the length of hospitalization without increasing the complication rate. |
|
29 |
Stephen George , Subaraman Ramchandran, Alexander Mihas , Kevin George , Ali Man- sour , Thomas Errico. |
Topical tranexamic acid reduc- es intra-operative blood loss and transfusion requirements in spinal deformity correction in patients with adolescent idi- opathic scoliosis(45). |
Topical TXA soaked sponges (1 g mixed in 500 ml Normal Saline) was utilized for wound packing during the entire surgical procedure. |
Spinal deformity correction in pa- tients with ado- lescent idiopathic scoliosis. |
The use of tTXA resulted in reduced operative blood loss, and blood transfusion requirements. |
|
30 |
Zhinan Ren , Shugang Li, Lin Sheng, Qianyu Zhuang, Zheng Li, Derong Xu, Xin |
Topical use of tranexamic acid can effectively decrease hidden blood loss during posterior lumbar spinal fusion surgery: A retrospective study(46). |
wound surface was soaked with TXA (1 g in 100 mL saline solution) for 5 min- utes before wound closure. |
posterior lumbar spinal fusion surgery. |
tTXA can effectively reduce HBL, without significant complications in adult patients undergoing pos- terior lumbar spinal fusion surgery. |
|
31 |
Wentao Wang , Kun Duan , Minjie Ma , Yong Jiang , Tuanjiang Liu , Jijun Liu , Dingjun Hao. |
Tranexamic Acid Decreases Visible and Hidden Blood Loss Without Affecting Prethrom- botic State Molecular Markers in Transforaminal Thoracic In- terbody Fusion for Treatment of Thoracolumbar Fracture- Dislocation(47). |
IV TXA. |
Transforaminal Thoracic Inter- body Fusion for Treatment of Thoracolumbar Fracture-Dislo- cation. |
TXA significantly reduced visible and hidden blood loss without affecting the prethrombosis-state mo- lecular markers in transfo- raminal thoracic interbody fusion or causing any nota- ble adverse effects. |
|
32 |
|
Tranexamic acid diminishes intraoperative blood loss and transfusion in spinal fusions for duchenne muscular dystro- phy scoliosis(48). |
TXA dose was 100 mg/ kg in solution over 15 minutes before incision followed by an infusion of 10 mg/kg per hour during surgery. |
Spinal fusions for duchenne mus- cular dystrophy scoliosis. |
TXA significantly reduces both intraoperative blood loss and the need for ho- mologous transfusion of whole blood and packed red blood cells in DMD patients undergoing posterior spinal fusion for scoliosis. |
|
33 |
Drew A Bednar , Vasiliki A Bednar, Ali Chaudhary, For- ough Farrokhyar. |
Tranexamic acid for hemos- tasis in the surgical treatment of metastatic tumors of the spine(49). |
IV TXA. |
surgical treat- ment of meta- static tumors of the spine. |
Estimated operative blood loss was 1385 mL in the study group treated with tranexamic acid and 1815 mL in controls not receiv- ing the drug, and was not found to be significantly decreased in this study. |
|
34 |
Derong Xu , Xin Chen , Zheng Li , Zhinan Ren , Qianyu Zhuang , Shugang Li. |
Tranexamic acid reduce hid- den blood loss in posterior lumbar interbody fusion (PLIF) surgery(50). |
The surgical field was im- mersed in TXA (1 g in 100 mL of saline solution) for 5 min before stitching the wound. |
Posterior lumbar interbody fusion (PLIF) surger. |
LIF is associated with mas- sive perioperative HBL, but the application of topical TXA leads to less postop- erative blood loss including less HBL, a lower blood product transfusion rate, and a shorter hospital stay for PLIF. |
|
35 |
Kristen E Jones , Elissa K But- ler, Tara Barrack , Charles T Ledonio , Mary L Forte, Claudia S Cohn , David W Polly Jr. |
Tranexamic Acid Reduced the Percent of Total Blood Volume Lost During Adolescent Idi- opathic Scoliosis Surgery(51). |
IV TXA. |
Adolescent Idi- opathic Scoliosis Surgery. |
Tranexamic acid significant- ly reduced the percentage of total blood volume lost versus no tranexamic acid in AIS patients who under- went PSF using a standard- ized blood loss measure. |
|
36 |
Linyu Yang , Xufeng Jia , Jian Yang , Jianping Kang. |
Tranexamic acid reduces blood cost in long-segment spinal fusion surgery: A randomized controlled study protocol(52). |
1000 mg of TXA mixed in 100 mL normal saline as a single dose intravenously over 20 minutes before the skin incision was made. |
Long-segment spinal fusion surgery. |
We hypothesized that TXA was effective and safe in reducing blood transfusion and cost in long-segment spinal fusion surgery. |
|
37 |
Takahiro Tsutsumimoto , Mit- suhiko Shimogata, Hiroshi Ohta, Mutsuki Yui, Isao |
Tranexamic acid reduces peri- operative blood loss in cervical laminoplasty: a prospective randomized study(53). |
15 mg/kg body weight of IV TXA. |
Cervical lamino- plasty. |
XA significantly reduced perioperative blood loss, primarily through a reduc- tion in postoperative blood loss, in cervical lamino- plasty. |
|
38 |
|
Tranexamic acid reduces perioperative blood loss of posterior lumbar surgery for stenosis or spondylolisthesis: A randomized trial(54). |
Bolus dose of 30 mg/kg TXA IV, a maintenance dosage of 2 mg/kg/h. |
Posterior lumbar surgery for ste- nosis or spondy- lolisthesis. |
TA significantly reduced the perioperative blood loss in patients undergoing poste- rior lumbar surgery for ste- nosis or spondylolisthesis. |
|
39 |
Fan, Yu Chen, Hailong Yu, Yan Bi, Zhengzhe Hua, Meihui Piao, Mingming Guo, Weijian Ren, Liangbi Xiang |
Tranexamic acid reduces post- operative blood loss of degen- erative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial(55). |
15 mg/kg body weight of IV TXA. |
Degenerative lumbar instabil- ity with stenosis in posterior ap- proach lumbar surgery |
Preoperative single-dose TXA can significantly re- duce postoperative blood loss in posterior approach lumbar surgery, and there were no significant side effects. |
|
40 |
Stefan Endres; Martin Heinz; Axel Wilke. |
Efficacy of Tranexamic Acid in Reducing Blood Loss in Poste- rior Lumbar Spine Surgery for Degenerative Spinal Stenosis With Instability(56). |
IV TXA. |
Posterior Lumbar Spine Surgery for De- generative Spinal Stenosis With Instability. |
On the basis of this retro- spective case control study, tranexamic acid use seems not to reduce transfusion rates in posterior lumbar spine surgery even if less blood loss was detectable. |
|
41 |
Xianren Zhu , Qian Shi , Dongya Li , Jibin Wu , Kaijin Guo , Xin Zheng , Hongwei Li. |
Two Doses of Tranexamic Acid Reduce Blood Loss in Primary Posterior Lumbar Fusion Sur- gery: A Randomized-controlled Trial(57). |
Group A was treated with 0.9% normal saline solu- tion without TXA, group B was treated with a 15 mg/kg loading dose intravenous infusion 30 minutes before surgery, and group C was treated with a 15 mg/kg loading dose intravenous infusion 30 minutes before sur- gery; then, the same dose was administered again 3 hours later. |
Primary Poste- rior Lumbar Fu- sion Surgery. |
Two doses of TXA sig- nificantly reduced the total blood loss, the hidden blood loss and postoperative drainage, and decreased hemoglobin and hematocrit drop in patients undergo- ing posterior lumbar fusion without increasing the risk of complications. |
|
42 |
Manee Raksakietisak , Benjab- horn Sathitkarnmanee, Peer- anat Srisaen, Tithiganya Duangrat, Thitima China- choti, Pranee Rushatamukaya- nunt, Nuchanat Sakulpacha- roen. |
Two Doses of Tranexamic Acid Reduce Blood Transfusion in Complex Spine Surgery: A Prospective Randomized Study(58). |
2 doses (15 mg/kg) of TXA. The first dose was administered before an- esthesia induction and the second dose, after 3 hours. |
Complex laminectomy (multilevel laminectomy or laminectomy and instrumenta- tion). |
2 effective doses (15 mg/ kg) of TXA can reduce blood loss and transfusions in low-risk adults undergoing complex spine surgery. |
|
43 |
Abhinandan Reddy Mallepal- ly , Rajat Mahajan , Tarush Rustagi , Shakti Amar Goel , Kalidutta Das , Har- |
Use of Topical Tranexamic Acid to Reduce Blood Loss in Single-Level Transforaminal Lumbar Interbody Fusion(59). |
The wound surface was soaked with TXA (1 g in 100 mL saline solution) for 3 minutes after exposure, after decompression, and before wound closure. |
Single-Level Transforaminal Lumbar Inter- body Fusion. |
Topical TXA is a viable, cost-effective method of decreasing perioperative blood loss in major spine surgery with fewer overall complications than other methods. Further studies are required to find the ideal dosage and timing. |
|
44 |
Liu, Xiangwang Huang , Wei Xiong , Hong Zhao , Sooyong Chua , Zheng Li. |
Using tranexamic acid soaked absorbable gelatin sponge following complex posterior lumbar spine surgery: A rand- omized control trial(60). |
TXA Soaked Gelfoam. |
Complex poste- rior lumbar spine surgery. |
TXA-soaked absorbable gelatin sponge is a safe, ef- fective treatment for reduc- tion of post-operative blood loss and blood transfusions among low-risk adult pa- tients undergoing lumbar spine surgery. |
DISCUSSION
44 Studies were investigated in this Systematic Review, Tranexamic Acid was used in Different Spine Surgeries that Included surgical correction for different types of scoliosis , single and multilevel ,instrumented and non-instrumented decompressive surgeries and on different levels, Surgical Fixation of the Spine for variable conditions including trauma, As well as Spinal fusion Surgeries on different levels and for variable conditions [17-60].TXA was administered in different methods , including IV, Topical And Combined ,different Doses were administered in different studies ,Different parameters were investigated in these Studies including intraoperative ,postoperative bleeding ,The need for Blood transfusion , the pre & Post-operative coagulation profile as well as parameters related to the safety profile of the Drug , Most of the studies supported the use of Tranexamic acid in spine surgeries in low doses , high doses , Topical use and combined use ,based on the resulted decreasing perioperative bleeding and the need for postoperative blood transfusion, and the relatively safe profile of TXA suggested by negligible encountered side effects and coagulation profile changes following administration[17-60], Other limited number of Studies didn’t Find any significant difference between groups that received placebo [39, 49].and TXA but we think are of No Statistical significance taking into account the large number of studies in this regard that supports the valuable effect Different doses and administration methods of TXA in decreasing perioperative bleeding and the need for postoperative blood transfusions [17-60].
CONCLUSION
As most of the patients that were included in these studies were otherwise healthy, and individuals that have chronic illnesses were excluded from those studies [17-60], So the effect of TXA may still need to be investigated in patients with Chronic illnesses like hypertension and diabetes mellitus along with other common disease entities that could be encountered in the daily practice of spine surgeries.
REFERENCES
1. Watts G. Utako Okamoto. Lancet.2016; 387: 2286.
2. Law RH, Wu G, Leung EW, Hidaka K, Quek AJ, et al. X-ray crystal structure of plasmin with tranexamic acid-derived active site inhibitors. Blood Adv. 2017; 1: 766-771.
3. Binz S, McCollester J, Thomas S, Miller J, Pohlman T, Waxman D, et al. CRASH-2 Study of Tranexamic Acid to Treat Bleeding in Trauma Patients: A Controversy Fueled by Science and Social Media. J Blood Transfus. 2015: 874920.
4. Melvin JS, Stryker LS, Sierra RJ. Tranexamic Acid in Hip and Knee Arthroplasty. J Am Acad Orthop Surg. 2015; 23: 732-740.
5. Engborn L, Blombäck M, Berntorp E. Tranexamic acid--an old drug still going strong and making a revival. Thromb Res. 2015; 135: 231-242.
6. Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, et al. Antifibrinolytic use for minimizing perioperative allogeneic blood transfusion. Cochrane Database Syst Re. 2011; 1: CD001886.
7. McEvoy M. Tranexamic Acid (TXA): Drug Whys. 2015: 1.
8. Andersson L, Nilsson IM, Niléhn JE, et al. Experimental and clinical studies on AMCA, the antifibrinolytically active isomer of p-aminomethyl cyclohexane carboxylic acid. Scand J Haematol. 1965; 2: 230-247.
9. Yang B, Li H, Wang D, et al. Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spinal surgery. PLoS One. 2013; 8: e55436.
10. Miyanji F Kilb B. University of British Columbia. Topical tranexamic acid in major paediatric spine deformity surgery: a randomized controlled trial. 2015.
11. British national formulary: BNF 69. 2015.
12. Cyklokapron Tablets - Summary of Product Characteristics (SPC) - (eMC). 2016.
13. Chornenki N, Um K, Mendoza P, Samienezhad A, Swarup V, et al. Risk of venous and arterial thrombosis in non-surgical patients receiving systemic tranexamic acid: A systematic review and meta-analysis. Thrombosis Research. 2019; 179: 81-86.
14. Tse E, Cheung W, Ng K, Luk K, et al. Reducing perioperative blood loss and allogeneic blood transfusion in patients undergoing major spine surgery. J Bone Joint Surg Am. 2011; 93: 1268-1277.
15. Zollo R, Eaton M, Karcz M, Pasternak R, Glance L, et al. Blood transfusion in the perioperative period. Best Pract Res Clin Anaesthesiol. 2012; 26: 475-484.
16. Hofmann A, Ozawa S, Farrugia A, Farmer L, Shander A, et al. Economic considerations on transfusion medicine and patient blood management. Best Pract Res Clin Anaesthesiol. 2013; 27: 59-68.
17. Carabini L, Moreland N, Vealey R, Bebawy J, Koski T, et al. A Randomized Controlled Trial of Low-Dose Tranexamic Acid versus Placebo to Reduce Red Blood Cell Transfusion During Complex Multilevel Spine Fusion Surgery. World Neurosurg. 110: e572-e579.
18. Sudprasert W, Tanaviriyachai T, Choovongkomol K, Jongkittanakul S, Piyapromdee U, et al. A Randomized Controlled Trial of Topical Application of Tranexamic Acid in Patients with Thoracolumbar Spine Trauma Undergoing Long-Segment Instrumented Posterior Spinal Fusion. Asian Spine J. 2019; 13: 146-154.
19. Yu C, Gao W, Yang j, Gu H, Zhu M, Sun K, et al. Can tranexamic acid reduce blood loss in cervical laminectomy with lateral mass screw fixation and bone grafting: a retrospective observational study. Medicine (Baltimore). 2017; 96: e6043.
20. Ou Y, Wei Y, Li R, Liang B, Qiu D, Wei M, et al. Clinical Research of Combined Intravenous Administration and Topical Application of Tranexamic Acid to a Surgical Wound During Posterior Lumbar Fusion. Surg Innov. 2018; 25: 128-135.
21. Ehresman J, Pennington Z, Schilling A, Medikonda R, Huq S, et al. Clinical Research of Combined Intravenous Administration and Topical Application of Tranexamic Acid to a Surgical Wound During Posterior Lumbar Fusion. Cost-benefit analysis of tranexamic acid and blood transfusion in elective lumbar spine surgery for degenerative pathologies. Neurosurg Spine. 2020; 20: 1-9.
22. Wang X , Yang R, Sun H , Zhang Y et al. Different Effects of Intravenous, Topical, and Combined Application of Tranexamic Acid on Patients with Thoracolumbar Fracture. World Neurosurg. 127: e1185-e1189.
23. Larson E, Evans T, Long J, Gannon E, Lyden E, et al. Does Prophylactic Administration of TXA Reduce Mean Operative Time and Postoperative Blood Loss in Posterior Approach Lumbar Spinal Fusion Surgery Performed for Degenerative Spinal Disease? Clin Spine Surg. 2019; 32: E353-E358.
24. Pradhan R, Pandey B, et al. The effect of tranexamic acid in blood loss and transfusion volume in adolescent idiopathic scoliosis surgery: a single-surgeon experience. JNMA J Nepal Med Assoc. 2015; 53: 169- 173.
25. Elmose S, Andersen M, Andresen E, Carreon L, Elmose S, et al. Double[1]blind, randomized controlled trial of tranexamic acid in minor lumbar spine surgery: no effect on operative time, intraoperative blood loss, or complications. J Neurosurg Spine. 2019: 1-7.
26. Yong H , Hyun S , Kim K, Jahng T , Kim H, et al. Effectiveness and Safety of Tranexamic Acid in Spinal Deformity Surgery. J Korean Neurosurg Soc. 2017; 60: 75-81.
27. Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, et al. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo[1]controlled study. Spine (Phila Pa 1976). 2008 Nov 15; 33: 2577-2580.
28. Ren Z, Zhuang Q, Li Z, Xu D, Chen X, et al. Efficacy and Safety of Topical Use of Tranexamic Acid in Reducing Blood Loss During Primary Lumbar Spinal Surgery: A Retrospective Case Control Study. Spine (Phila Pa 1976). 2017; 42: 1779-1784.
29. Farrokhi M, Kazemi A, Eftekharian H, Akbari K, et al. Efficacy of prophylactic low dose of tranexamic acid in spinal fixation surgery: a randomized clinical trial. J Neurosurg Anesthesiol. 2011; 23: 290-296.
30. Endres S, Heinz M, Wilke A, et al. Efficacy of tranexamic acid in reducing blood loss in posterior lumbar spine surgery for degenerative spinal stenosis with instability: a retrospective case control study. BMC Surg. 2011; 11: 29.
31. 31- Lieberman L, High-Dose Tranexamic Acid Reduces Blood Loss in Extensive Spine Surgery. 2021(cited 2020 march 1); 1(1): (2 screens) available from URL: https://www.spineuniverse.com/professional/ news/high-dose-tranexamic-acid-reduces-blood-loss-extensive[1]spine-surgery# accessed on 20-3-2021.
32. Kushioka J, Yamashita T, Okuda S, Maeno T, Matsumoto T, Yamasaki R, et al. High-dose tranexamic acid reduces intraoperative and postoperative blood loss in posterior lumbar interbody fusion. J Neurosurg Spine. 2017; 26: 363-367.
33. M J Colomina, M Koo, M Basora, J Pizones, L Mora, et al. Intraoperative tranexamic acid use in major spine surgery in adults: a multicenter, randomized, placebo-controlled trial. Br J Anaesth. 2017; 118: 380- 390.
34. Shakeri M, Salehpour F, Shokouhi G, Aeinfar K, Aghazadeh J, Mirzaei F, et al. Minimal Dose of Tranexamic Acid Is Effective in Reducing Blood Loss in Complex Spine Surgeries: A Randomized Double-Blind Placebo Controlled Study. Asian Spine J. 2018; 12: 484-489.
35. Xie J, Lenke L, Li T, Si Y, Zhao Z, Wang Y, et al. Preliminary investigation of high-dose tranexamic acid for controlling intraoperative blood loss in patients undergoing spine correction surgery. Spine J. 2015; 15: 647-654.
36. Zhang H, Dong L,Wang H, Hu F, Shao Q, et al. Safety and efficacy of tranexamic acid in spinal canal tumors: a retrospective cohort study. Br J Neurosurg. 2020; 34: 313-315.
37. Perez-Roman R, Lugo-Pico J , Burks j , Madhavan K , Sheinberg D, et al. Short-term safety of tranexamic acid use in posterior cervical decompression and fusion surgery. J Clin Neurosci. 2019; 66: 41-44.
38. Zhang Z, Wang L , Yang X, Xiu L , Zhou Z, et al. The effect of multiple-dose oral versus intravenous tranexamic acid in reducing postoperative blood loss and transfusion rate after adolescent scoliosis surgery: a randomized controlled trial. Spine J. 2021; 21: 312-320.
39. Haddad A, Ames C, Safaee M, Deviren V, Lau D, et al. Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Global Spine J. 2020 Sep 24; 2192568220953812.
40. Lykissas M, Crawford A, Chan G, Aronson L, Al-Sayyad M. The effect of tranexamic acid in blood loss and transfusion volume in adolescent idiopathic scoliosis surgery: a single-surgeon experience. J Child Orthop. 2013; 7: 245-249.
41. Hong S, Luoyi D, Jin D, Jian W, Hao Z, et al. The Efficacy and Safety of Prophylactic Intravenous Tranexamic Acid on Perioperative Blood Loss in Patients Treated with Posterior Lumbar Interbody Fusion. World Neurosurg. 2019; 125: e198-e204.
42. Raksakietisak M, Sathitkarnmanee B, Srisaen P, Duangrat T, Chinachoti T, et al. The efficacy and safety of tranexamic acid in reducing perioperative blood loss in patients with multilevel thoracic spinal stenosis: A retrospective observational study”. Medicine (Baltimore). 2018; 97: e13643.
43. Visagie M, Qin C, Cho B ,Merkel K, Kajstura T, Raj A , Purvis T, et al. “The impact of patient blood management on blood utilization and clinical outcomes in complex spine surgery. Transfusion. 2019; 59: 3639-3645.
44. Tian-Hua R , Zhe Liu H, Ming-Sheng T , Hong Z , Xiang-Yang L, et al. Topical Injection of tranexamic Acid via a Drain Plus Drain-Clamping to Reduce Blood Loss in Degenerative Lumbar Scoliosis Surgery. Clin Spine Surg. 2020; 100: e356.
45. Stephen G, Subaraman R, Alexander M , Kevin G, Mansour A, et al. Topical tranexamic acid reduces intra-operative blood loss and transfusion requirements in spinal deformity correction in patients with adolescent idiopathic scoliosis” Spine Deform. 2021; 50: e240- 244
46. Zhinan R, Shugang L, Lin S, et al. Topical use of tranexamic acid can effectively decrease hidden blood loss during posterior lumbar spinal fusion surgery: A retrospective study Medicine (Baltimore). 2017; 96: e8233.
47. Wentao W, Kun D, Minjie M, Yong J, Tuanjiang L, et al. Tranexamic Acid Decreases Visible and Hidden Blood Loss Without Affecting Prethrombotic State Molecular Markers in Transforaminal Thoracic Interbody Fusion for Treatment of Thoracolumbar Fracture[1]Dislocation. Spine (Phila Pa 1976). 2018; 43: E734-E739.
48. Shapiro F, Zurakowski D, et al. Tranexamic acid diminishes intraoperative blood loss and transfusion in spinal fusions for duchenne muscular dystrophy scoliosis. Spine(Phila Pa 1976). 2007; 32: 2278-2283.
49. Drew B, Vasiliki B, Ali C, Forough F, et al. Tranexamic acid for hemostasis in the surgical treatment of metastatic tumors of the spine. Spine (Phila Pa 1976). 2006; 31: 954-957
50. Derong X, Xin C, Zheng L, Zhinan R, Qianyu Z, et al. Tranexamic acid reduce hidden blood loss in posterior lumbar interbody fusion (PLIF) surgery. Medicine (Baltimore). 2020; 99: e19552.
51. Kristen J, Elissa B, Tara B, Charles L, Mary F, et al. Tranexamic Acid Reduced the Percent of Total Blood Volume Lost During Adolescent Idiopathic Scoliosis Surgery. Int J Spine Surg. 2017; 11: 27.
52. Linyu Y, Xufeng J, Jian Y, Jianping K, et al. Tranexamic acid reduces blood cost in long-segment spinal fusion surgery: A randomized controlled study protocol. Medicine (Baltimore). 2020; 99: e22069
53. Takahiro T, Mitsuhiko H, et al. Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine (Phila Pa 1976). 2011; 36: 1913-1918.
54. Houyin S, Yunsheng O, Dianming J, Zhengxue Q, Zenghui Z, et al. Tranexamic acid reduces perioperative blood loss of posterior lumbar surgery for stenosis or spondylolisthesis: A randomized trial. Medicine (Baltimore). 2017; 96: e5718.
55. Qi W , Jun L, Rong F, Yu C, Hailong Y, Yan B, et al. Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Eur Spine J. 2013; 22: 2035-2038.
56. Stefan E, Martin H, et al. Efficacy of Tranexamic Acid in Reducing Blood Loss in Posterior Lumbar Spine Surgery for Degenerative Spinal Stenosis With Instability. BMC Surg. 2011; 11: e233-234.
57.Xianren Z , Qian S , Dongya L , Jibin W , Kaijin G, et al. Two Doses of Tranexamic Acid Reduce Blood Loss in Primary Posterior Lumbar Fusion Surgery: A Randomized-controlled. Trial Clin Spine Surg. 2020 ; 33: E593-E597.
58.Manee R , Benjabhorn S, Tithiganya D, Thitima C, , Nuchanat S, et al. Two Doses of Tranexamic Acid Reduce Blood Transfusion in Complex Spine Surgery: A Prospective Randomized Study. Spine (Phila Pa 1976). 2015 ; 40: E1257-1263.
59. Abhinandan M, Rajat M , Tarush R , Shakti A , Kalidutta D , et al. Use of Topical Tranexamic Acid to Reduce Blood Loss in Single-Level Transforaminal Lumbar Interbody Fusion. Asian Spine J. 2020; 14: 593-600.
60. Jinqian L , Hongzhe L, Xiangwang H , Wei X , Hong Z , et al. Using tranexamic acid soaked absorbable gelatin sponge following complex posterior lumbar spine surgery: A randomized control trial. Clin Neurol Neurosurg. 2016; 147: 110-114.