The Role of IDH1 Gene Expression in Breast Cancer: Insights into Tumor Progression and Metastasis
- 1. Department of Cell and Molecular Sciences, Tehran Medical Sciences, Islamic Azad University, Iran
- 2. Department of Physiology, Islamic Azad University, Iran
- 3. Applied Biotechnology Research Center, Tehran Medical Sciences, Islamic Azad University, Iran
ABSTRACT
Breast cancer is the 2nd most common cancer worldwide after lung cancer and the first most common cancer among women. BRCA1, BRCA2, HER2, and IDH1 are important genes that play a prominent role in breast cancer. Understanding gene expression involves investigating various molecular pathways and metabolic processes. In this review, we have focused on the isocitrate dehydrogenase (IDH1) gene’s role in cancer progression, especially in breast cancer and glioma. IDH-mutant tumors can alter DNA methylation, influencing tumor progression and the microenvironment. Although IDH1 expression is reduced in breast cancer, its expression in metastatic breast cancer is higher compared to non-metastatic breast cancer. We also explained the prevalence of IDH1 in different grades and stages of breast cancer, highlighting its clinical implications for diagnosis, prognosis, and potential targeted therapies.
KEYWORDS
- Breast Cancer
- IDH1
- Gene Expression
INTRODUCTION
Overview of breast cancer
Breast cancer is a common malignant tumor affecting women, arising from various internal and external factors [1-3]. Lifestyle, environment, poor lifestyle choices, and social-psychological factors contribute to its occurrence. Environmental influences and social-psychological factors contribute to breast cancer. Genetic mutations and family history account for 5% to 10% of cases, while 20% to 30% are linked to modifiable factors. Age, family history, and environmental elements further complicate the causes of breast cancer [4,5].
The importance of gene expression in tumor biology
Gene expression regulation is essential for p53’s tumor- suppressive action. The mechanisms by which p53 activates gene programs to prevent tumor development, along with how these pathways are altered without inactivating mutations, remain largely unexplored. Through regulating specific gene networks, p53 inhibits the initiation and progression of tumors. Mutations in IDH associated with cancer can disrupt p53’s tumor-suppressive functions by modifying chromatin structures that control gene expression. Mutant IDH alters chromatin accessibility, limiting the activation of key pro-apoptotic p53 targets such as the death receptor Fas, crucial for p53-dependent tumor suppression. In TP53 wild-type cholangiocarcinoma cells-where neither p53 nor IDH mutations are present-blocking mutant IDH enhances the expression of p53-targeted genes, increasing cell susceptibility to Fas ligand- induced apoptosis and chemotherapy. This disruption of p53 target gene regulation by IDH mutations represents a reversible cancer-associated effect [6].
IDH1 gene: structure and function
IDH exists in three human isoforms: IDH1, IDH2, and IDH3 [7]. IDH1 (cytosolic) and IDH2 (mitochondrial) are NADP+-dependent and play roles in tumorigenesis, while IDH3 (mitochondrial) is NAD+-dependent and contributes to the citric acid cycle [8]. Mutant IDH1 predominantly generates 2-hydroxyglutarate (2-HG), an oncometabolite that promotes tumor development through various downstream pathways [9].
IDH1 Structure: The IDH1 gene, located on chromosome 2q34, spans 10 exons across 18.9 kb [10,11]. It encodes the IDH1 protein, comprising 414 amino acids with a molecular weight of 46.7 kD, localized in the cytoplasm and peroxisomes [9,12]. IDH1 forms a homodimer with two hydrophilic active sites and two protein subunits. Each monomer includes a large domain, a small domain, and a clasp domain with two clefts, creating an active NADP+ binding site through the interaction of the large and small domains of one monomer with the small domain of the other monomer [13].
IDH1 Function: IDH1 facilitates the oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG), a process that depends on NADP+ and produces NADPH [12,14]. This reversible reaction supports cellular functions such as lowering oxidative stress through cytoplasmic NADPH production and maintaining DNA and histone demethylation by α-KG. (13,15,16), IDH1-generated NADPH is crucial for cholesterol and fatty acid biosynthesis and plays a role in glucose-stimulated insulin release [11,17]. Under hypoxic conditions, α-KG is converted to acetyl-coenzyme A, necessary for lipogenesis [18].
Molecular pathways involved in IDH1 gene expression
Understanding the complex interactions between IDH mutations and other molecular pathways in cancer is essential for developing targeted therapies to slow tumor progression and enhance patient outcomes [19]. About tumorigenesis linked to IDH, wild-type IDH1/2 (wtIDH1/2) transforms isocitrate from the citric acid cycle into α-KG, generating NADPH. In contrast, mutant IDH1/2 (mutIDH1/2) converts α-KG into the oncometabolite R-2-HG, which consumes NADPH. R-2-HG inhibits the α-KG- dependent dioxygenase family, leading to epigenetic changes via the inhibition of histone lysine demethylases (KDM) and the 5-methylcytosine hydroxylase TET2. This inhibition also disrupts collagen maturation by affecting prolyl-hydroxylation processes. Additionally, R-2-HG has been shown to activate prolyl-hydroxylase 2 (PHD2), which inhibits hypoxia-inducible factor 1-alpha (HIF-1α), although some studies indicate it may also exert an inhibitory effect [20,21].The mutant variant of IDH could alter the cellular redox state by adjusting the ratio of NADPH to NADP+ [22].
IDH1 role in oxidative stress and metabolism
Introducing cytotoxic oxidative stress is proposed as a viable therapeutic strategy for various cancers [23]. Elevated ROS levels inhibit cancer cell growth and induce cell death. Despite this, IDH1-mutated cells survive high ROS levels by leveraging intrinsic antioxidant pathways to mitigate oxidative stress [24].
IDH1 is essential in cellular metabolism, maintaining redox balance, regulating epigenetics, and facilitating DNA repair [25]. Mutations in IDH1 contribute to the development and progression of various cancers by causing excessive production of D-2-hydroxyglutarate (D-2-HG), which hinders cellular maturation and normal function, ultimately leading to cancer [26-28].This approach has led to the creation of pharmacological inhibitors aimed at mutant IDH for cancer treatment. However, current research primarily focuses on inhibiting IDH mutations, overlooking the tumor suppressor role of wild-type IDH1. The impact of IDH1 on tumor cell growth varied based on the cultural environment. In normal conditions, overexpression or activation of IDH1 inhibited tumor cells, potentially linked to its regulation of HIF1a stability [29-31].
IDH1 supports tumor cell growth under low glucose or nutrient deficiency, potentially by facilitating amino acid utilization and managing oxidative stress [32,33]. Cancer cells in solid tumors interact with a complex microenvironment, influenced by the vascular system’s condition. Therefore, understanding IDH1’s role in tumor progression under different circumstances is essential. A key characteristic of tumor cells is the Warburg effect, marked by increased glucose consumption, active glycolysis, and elevated lactic acid metabolism [34]. The HIF1a signaling pathway is significant in regulating aerobic glycolysis [35].
The impact of IDH1 mutations in cancer progression
IDH1 and IDH2 play crucial roles in essential metabolic functions in human cells, influencing differentiation, proliferation, and the response to oxidative damage. IDH mutations have been linked to the advancement and development of tumors in various solid tumors, including glioma, cholangiocarcinoma, and chondrosarcoma, among others, and have become significant markers for molecular classification and prognostic evaluation [36].The acquisition of IDH mutations is a significant event in the progression of many tumors, and targeting these mutations early in the disease can be vital to interrupt disease advancement and avoid further genetic alterations that could diminish the efficacy of IDH inhibitors [37].
Treatment of IDH-mutant tumors can lead to a loss of DNA methylation, which triggers the epigenetic activation of genes associated with early tumor advancement and changes in the tumor microenvironment’s composition, shifting towards angiogenesis and a T-cell profile that mirrors a treatment- naive IDH-wildtype glioma. In untreated IDH mutation patients, the epigenome is relatively stable, resulting in later tumor progression compared to those who have been treated, either spontaneously or through subsequent therapies [38].
Correlation of IDH1 expression with breast cancer metastasis
IDH1 expression levels were notably reduced in breast cancer tissues compared to neighboring normal tissues, indicating that IDH1 is essential in influencing breast cancer metastasis. Additionally, reduced IDH1 expression was strongly linked to a worse prognosis in breast cancer patients [39].
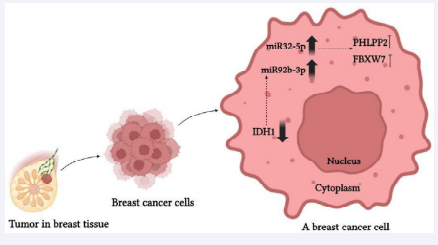
The decreased expression of IDH1 was due to the abnormal overexpression of miR-32-5p and miR-92b-3p in breast cancer. miR-32-5p contributes to an oncogenic role by enhancing the proliferation and invasive potential of breast cancer cells through the direct inhibition of PHLPP2 and FBXW7 [40,41] (Figure 1).
Figure 1 In breast cancer cells, by reducing the expression of IDH1, miR-32-5p and miR-92b-3p are overexpressed, and the increased expression of miR-32-5p plays an inhibitory role for PHLPP2 and FBXW7.
The expression levels of the IDH1 gene were markedly elevated in patients diagnosed with metastatic breast cancer
and invasive ductal carcinoma when compared to those who did not exhibit metastatic characteristics. As the tumor’s degree and stage escalated, there was also a corresponding rise in IDH1 gene expression within more closely related samples. In the initial phases of the tumor, cancer cells can inhibit the expression of the IDH1 gene, thereby decreasing the redox balance and elevating HIF-1α levels, which facilitates metastasis and aids in their growth and development. However, as cancer progresses, elevated IDH1 expression may boost the energy supply to the cancer cells through enhanced metabolism, further driving cancer progression. Thus, monitoring the elevation of this gene’s expression can be useful for understanding the advancement and prognosis of the disease [42,43].
Clinical implications of IDH1 in metastatic and non- metastatic breast cancer
IDH1 is being explored as a therapeutic target in cancers with the mutated enzyme (mtIDH1). Small molecule inhibitors of IDH1, such as ivosidenib, show significant biological activity in tumors with mutant IDH1. They are remarkably well tolerated by patients. Identifying the role of wild-type IDH1 in these and other cancers, understanding the mechanisms underlying resistance to IDH1 inhibitors, and exploring synergistic treatment combinations are important next steps [44].
IDH2 is linked to a more aggressive form of breast cancer by enhancing cell proliferation, which is distinct from the roles of IDH1 and IDH3α. Additionally, the immunohistochemical evaluation of IDH2 serves as a significant prognostic indicator, particularly for patients with ER-positive breast cancer [45].
IDH1 expression patterns in different stages and grades of breast cancer
A study examined the relationship between IDH1 expression and clinicopathological characteristics in patients with breast invasive ductal carcinoma [39]. IDH1 levels were found to be 77.2% in well-differentiated and moderately differentiated grades, compared to 22.8% in poorly differentiated grades, indicating higher expression in the well- differentiated and moderately differentiated grades than poorly differentiated. In pathological stages, IDH1 expression was 15.7% in stage one, 49.1% in stage two, and 35.2% in stage three. For the pT stage, IDH1 levels were 25.1% for T1, 64.4% for T2, and 10.5% for T3 and T4. In the pN stage, IDH1 was measured at 40.4% in N0, 26.6% in N1, 22.8% in N2, and 10.1% in N3, suggesting a decrease in IDH1 levels with greater lymph node involvement. Regarding vascular invasion, IDH1 levels were 62.9% without vascular invasion and dropped to 37.1% in its presence, indicating higher IDH1 expression in the absence of vascular invasion (Table 1).
Table 1: IDH1 percentage across various stages and grades in breast cancer. Diagnostic and prognostic value of IDH1 in breast cancer
|
Variables |
IDH1% |
|
Grading
|
77.2 22.8 |
|
Pathology stage
|
15.7 49.1 35.2 |
|
pT stage
|
25.1 64.4 10.5 |
|
pN stage
|
40.4 26.6 22.8 10.1 |
|
Vascular invasion
|
62.9 37.1 |
In metabolism and energy production, the IDH family has a vital role. The relative expression levels of all three IDH isoforms have prognostic significance in many cancers, like breast cancer [46]. Accurate prognosis and treatment for this cancer heavily depend on cytogenetic and molecular diagnosis. The use of molecular markers like the IDH1 gene has significantly improved prognostic accuracy, allowing for predictions regarding potential metastasis [47].
Overexpression of miR-92b3p and miR-32-5p led to tumor- suppressive silencing of IDH1 in breast cancer tissues compared to adjacent normal tissues. IDH1 depletion in breast cancer cells accelerates migration and invasion by activating snail expression. Low IDH1 levels, especially in conjunction with snail expression, may serve as a prognostic biomarker for breast cancer [39]. IDH1 may play a role in the invasion, migration, and growth of breast cancer cells, with low IDH1 expression associated with poor patient prognosis. Reduced expression levels of IDH1, instead of a mutation in IDH1, significantly impact the prognosis of breast cancer [39,48].
Comparative studies: IDH1 in breast cancer vs. other cancers
Alterations in the genes responsible for IDH1 and IDH2, and to a lesser extent IDH3, have been identified in various cancers, including glioma, acute myeloid leukemia (AML), chondrosarcoma, and intrahepatic cholangiocarcinoma. Mutations affecting the catalytic domain of IDH can cause significant disruptions in the cell cycle, but they may also lead to the production of oncometabolites that can encourage abnormal cellular growth, ultimately contributing to the onset of cancer [19]. IDH mutations have also been infrequently found in prostate cancers, paragangliomas, and melanomas [49-51].
AML, chondrosarcoma, and cholangiocarcinoma are found in the liver and intrahepatically. The mutated protein loses its usual enzymatic function and acquires the ability to produce 2-HG, a compound harmful to life. 2-HG competes with enzymes, inhibiting α-KG-dependent cells vital for gene regulation and tissue stability. The expression of mutated IDH disrupts cell differentiation across various cell lines and encourages tumor progression alongside other cancer- related genes [22].
Tumor development in prostate cancer is driven by a multifaceted interaction between androgen receptors (AR) and the activity of extra-mitochondrial IDH1, showing therapies aimed at IDH1 can be a viable treatment strategy [52,53].
The expression levels of the IDH1 gene in breast cancer tissue are notably lowered. The IDH1 enzyme generates NADPH by converting isocitrate into α-KG, acting as a key reducing agent for the regeneration of glutathione (GSH), through GSH reductase and the NADPH-dependent thioredoxin system, both of which are crucial for protecting cells against oxidative damage. IDH1 is capable of producing NADPH in the cytosol of cells, suggesting that it aids in the defense against oxidative stress caused by reactive oxygen species. Consequently, any disruption to the IDH1 enzyme and gene can lead to an imbalance in redox status by diminishing NADPH production, which may be advantageous for cancer cells. The IDH1 gene has demonstrated greater expression levels in individuals with metastatic breast cancer compared to those with non-metastatic breast cancer [39,42].
IDH1 expression in gliomas
Glutamine and/or glutamate are crucial substrates that enhance lipid and glutathione synthesis to mitigate metabolic impacts. Notably, IDH-mutated gliomas exhibit a unique metabolic profile characterized by significantly reduced glycolysis, a hallmark of rapidly proliferating tumors, in contrast to other solid tumors. The distinctive metabolic processes in IDH-mutated gliomas not only clarify the slower growth rate of this type of cancer but also indicate that creating targeted strategies focused on the specific metabolic characteristics of IDH mutants could be a promising direction for future glioma treatments [54]. IDH mutations have emerged as key therapeutic targets for IDH-mut diffuse glioma. Two different approaches are being rigorously tested in clinical trials: 1. Diminishing the level of intratumoral 2-HG by directly inhibiting the function of the mutated IDH enzyme; 2. Exploiting the cellular weaknesses resulting from 2-HG accumulation [55].
The role of 2-Hydroxyglutarate in tumorigenesis
Mutations in IDH1/2 may disrupt cellular redox reactions, fostering tumor development [56]. 2-HG is a chiral compound, which has D- and L-enantiomer forms. 2-HG facilitates tumor development by blocking endostatin, resulting in increased tumor vascularization and proliferation [57]. Although cancer cells predominantly generate D- 2-HG, L-2-HG exhibits stronger inhibition of multiple enzymes in vitro and has a more significant impact on cell proliferation [20,21,58]. Although IDH1/2 mutations have diverse effects on tumor development, the strong association between elevated levels of 2-HG in gliomas has prompted extensive research into the particular influence of 2-HG on the formation of gliomas [57].
Histone methylation serves as a crucial epigenetic mechanism in cancer development, highlighting the role of 2-HG- induced histone methylation in glioma formation. The relevance of histone methylation in tumor development is further supported by numerous studies indicating that histone methylation hampers cellular differentiation [59]. DNA methylation plays a crucial role in tumor development, as cancer cells frequently show irregular DNA methylation patterns, such as widespread DNA hypomethylation and hypermethylation of promoters. These changes are pro-tumorigenic: hypomethylation contributes to genomic instability, whereas promoter hypermethylation, among other effects, results in the silencing of tumor suppressor genes [60]. 2-HG facilitates tumor development by hindering the repair of DNA damage caused by alkylation, acting as a competitive inhibitor of AlkB proteins [57].
Therapeutic potential of targeting IDH1
The distinct behaviors associated with IDH mutations produce specific patterns in cancer metabolism, alterations in epigenetics, and resistance to treatment. Innovative molecular targeting strategies have been created to enhance the effectiveness of treatments for cancers with IDH mutations [54].
The initial synthetic inhibitor targeting the IDH mutation, AGI-5198, prevents the formation of D-2-HG and hinders the growth of IDH1-mutated xenografts in vivo. The second generation of inhibitors for IDH mutations, ivosidenib (AG-120), and vorasidenib (AG-881), have received approval from the Food and Drug Administration as a treatment option for IDH-mutated AML [61]. Numerous clinical studies are finished or in progress to assess the safety and effectiveness of ivosidenib and vorasidenib for treating IDH-mutated myeloid cancers and solid tumors, such as glioma [62].
D-2-HG inhibits DNA repair pathways by blocking enzymes such as AlkB homolog 2/3 (ALKBH2/3) and inhibiting the homologous recombination (HR) DNA repair mechanism [63- 65]. Inhibiting general DNA repair pathways may make residual DNA repair enzymes vulnerable in IDH-mutated cells, presenting a potential therapeutic strategy. Thus, combining small-molecule inhibitors that target poly-ADP ribose polymerase (PARP) could offer an effective treatment for IDH-mutated malignancies [65- 67].
IDH mutations correlate with therapeutic sensitivity, and targeting DNA repair enzymes is more effective in IDH- mutated cells; however, IDH-mutated gliomas may develop distinct DNA repair pathways compared to IDH wild- type gliomas [68,69]. Identifying modified DNA repair pathways in IDH-mutated glioma links cancer metabolism to genomic instability, indicating therapeutic vulnerabilities in IDH-mutant cancers [54]. Mutations in IDH1 and IDH2, along with D2HG accumulation, may contribute to immune suppression in gliomas. Targeting mutant IDH enzymes could improve the efficiency of immunotherapeutic agents. Moreover, these mutations can modify the expression of immunogenic cytokines and receptors, leading to immunosuppression. Conversely, neoantigens specific to mutant IDH can elicit anti-tumor immune responses when triggered by peptide vaccination [70].
IDH-targeted immunotherapeutic strategies and agents that capitalize on the metabolic and epigenetic vulnerabilities linked to the IDH mutant phenotype face challenges due to the lack of reliable preclinical models that accurately reflect the genetic landscape, tumor microenvironment, and growth patterns of IDH-mutated low-grade gliomas (LGGs). This gap complicates the study of IDH mutations’ role in glioma development and IDH inhibitors’ therapeutic efficacy evaluation. An alternative approach is to exploit, rather than reverse, the IDH-mutant phenotype by targeting the lasting epigenetic and metabolic vulnerabilities associated with IDH mutations, even in advanced tumor stages [55].
Small molecule inhibitors of IDH1
The mutation in IDH1 results in an accumulation of 2-HG, which disrupts cellular differentiation, modifies gene expression, and causes epigenetic imbalances. An emerging therapeutic approach focuses on small-molecule inhibitors that specifically target mutant IDH1. A recent breakthrough in this field is the FDA approval of AG-120 (ivosidenib), a selective inhibitor for mutant IDH1, specifically for treating acute myeloid leukemia (AML) with IDH1 mutations [71].
AG-120 is a small-molecule drug designed to inhibit the IDH1/R132H mutation [72]. Although several small molecules targeting mutant IDH1 are under clinical investigation, AG-120 remains the only one with FDA approval. Many other candidates face limitations, such as poor druggability and limited efficacy in laboratory tests. Additionally, these compounds often suffer from challenges in pharmacokinetics, weak cellular potency, and low selectivity [71].
Small molecules are structured to attach to the active site of mutant IDH1, blocking the conformational shift necessary for converting α-KG to 2-HG [73]. By targeting mutant IDH1, these inhibitors lower the levels of 2-HG in cells and serum, counteracting the extensive epigenomic changes associated with the mutation. Selective inhibitors like AG-120 and IDH305 suppress mutant IDH activity, promoting cell differentiation in both laboratory and animal models [72,74-76].
Through virtual screening of comparative structures, new compounds such as L806-0255, V015-1671, and AQ- 714/41674992 have been identified as potential IDH1 inhibitors [77]. Among these, AQ-714/41674992 exhibited the highest inhibitory activity in enzyme assays. These compounds bind to hydrophobic residues within the binding pocket (PDB IDs: 5TQH and 6B0Z), forming stabilizing intermolecular hydrogen bonds with critical residues. L806-0255 and V015-1671 form key hydrogen bonds with ILE128, while V015-1671 and AQ- 714/41674992 interact with ALA111. Additional hydrophobic interactions with residues like VAL276, SER278, SER287, ILE128, and PRO118 enhance the binding affinity of these inhibitors to the mutant IDH1 R132H variant. AQ-714/41674992 shows strong potential as a novel IDH1 inhibitor, though further structural refinement is needed [71].
Future research directions for IDH1 in breast cancer
IDH1 gene expression changes may impact breast cancer prognosis; hence, further research is needed for definitive conclusions [42]. Increasing evidence suggests that metabolic alterations linked to IDH1 mutations may influence tumor microenvironment and immune response in breast cancer; these interactions can lead to different treatment methods for patients. Understanding the intricate role of IDH1 in breast cancer pathology is crucial, as it may reveal new therapeutic targets. Further investigation will enhance our understanding of the molecular mechanisms and may ultimately inform clinical decision-making.
CONCLUSION
IDH1 expression is reduced in breast cancer tissues and is linked to patient prognosis. This reduction is caused by the overexpression of miR-32-5p and miR-92b-3p, which promote cancer cell proliferation and invasion. IDH1 levels in metastatic breast cancer are elevated compared to non-metastatic cases.
Increased IDH1 expression correlates with tumor progression, with early stages suppressing the gene and later stages benefiting from elevated expression for increased energy supply. Monitoring IDH1 levels is essential for understanding cancer progression and prognosis, especially its critical role in breast cancer metastasis.
IDH mutations in cancer impact metabolism, epigenetics, and treatment resistance. Targeted molecular strategies enhance treatment efficacy for IDH-mutated cancers. These mutations affect therapeutic sensitivity, and DNA repair enzyme targeting is more successful in IDH-mutated cells. IDH-mutated gliomas may develop unique DNA repair pathways. Understanding these pathways can reveal vulnerabilities for therapeutic intervention. Mutant IDH enzymes contribute to immune suppression in gliomas but could be targeted to enhance immunotherapy efficiency. Further studies and clinical trials are crucial to determining the impact of IDH1 expression on long-term prognosis and assessing its potential as a biomarker for personalized treatment.
REFERENCES
- Obeagu EI, Babar Q, Vincent C, Udenze CL, Eze R, Okafor CJ, et al. Therapeutic targets in breast cancer signaling: a review. J Pharmaceut Res Int. 2021; 33: 82-99.
- Aizaz M, Khan M, Khan F, Munir A, Ahmad S, Obeagu E. Burden of breast cancer: developing countries perspective. Int J Innovative Appl Res. 2023; 11: 31-37.
- Ibekwe AM, Obeagu EI, Ibekwe CE, Onyekwuo C, Ibekwe CV, Okoro AD, et al. Challenges of exclusive breastfeeding among working class women in a teaching hospital South East, Nigeria. J Pharmaceut Res Int. 2022; 34: 1-10.
- Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, et al. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017; 13: 1387.
- Obeagu EI, Obeagu GU. Breast cancer: A review of risk factors and diagnosis. Med. 2024; 103: e36905.
- Martin CP, Sullivan WB, Brinkman J, Scoville DM, Yashinskie JJ, Tian S, et al. Mutant IDH uncouples p53 from target gene regulation to disable tumor suppression. bioRxiv. 2024.
- Dalziel K. Isocitrate dehydrogenase and related oxidative decarboxylases. FEBS lett. 1980; 117: K45-K55.
- Cairns RA, Iqbal J, Lemonnier F, Kucuk C, De Leval L, Jais JP, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012; 119: 1901-1903.
- Bruce-Brand C, Govender D. Gene of the month: IDH1. J Clin Pathol. 2020; 73: 611-615.
- Creagan R, Carritt B, Chen S, Kucherlapati R, McMorris F, Ricciuti F, et al. Chromosome assignments of genes in man using mouse-human somatic cell hybrids: Cytoplasmic isocitrate dehydrogenase (IDH 1) and malate dehydrogenase (MDH 1) to chromosomes 2. Am J Hum Genet. 1974; 26: 604.
- Shechter I, Dai P, Huo L, Guan G. IDH1 gene transcription is sterol regulated and activated by SREBP-1a and SREBP-2 in human hepatoma HepG2 cells: evidence that IDH1 may regulate lipogenesis in hepatic cells. J lipid Res. 2003; 44: 2169-2180.
- Geisbrecht BV, Gould SJ. The human PICD gene encodes a cytoplasmic and peroxisomal NADP+- dependent isocitrate dehydrogenase. J Biol Chem. 1999; 274: 30527-30533.
- Xu X, Zhao J, Xu Z, Peng B, Huang Q, Arnold E, et al. Structures ofhuman cytosolic NADP- dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem. 2004; 279: 33946-33957.
- Koshland Jr D, Walsh K, LaPorte D. Sensitivity of metabolic fluxes to covalent control. Current topics in cellular regulation. Elsevier. 1985; 13-22.
- Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim Biophys Acta. 2014; 1846: 326-341.
- Lee SM, Koh HJ, Park DC, Song BJ, Huh TL, Park JW. Cytosolic NADP+- dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radical Biol Med. 2002; 32: 1185-1196.
- Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, et al. A pyruvate cycling pathway involving cytosolic NADP- dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006; 281: 30593-30602.
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012; 481: 380-384.
- Ivanov S, Nano O, Hana C, Bonano-Rios A, Hussein A. Molecular Targeting of the Isocitrate dehydrogenase pathway and the implications for Cancer Therapy. Int J Mol Sci. 2024; 25: 7337.
- Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, Rose NR, et al. The oncometabolite 2- hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011; 12: 463-469.
- Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate- dependent dioxygenases. Cancer Cell. 2011; 19: 17-30.
- Clark O, Yen K, Mellinghoff IK. Molecular pathways: isocitrate dehydrogenase mutations in cancer. Clin Cancer Res. 2016; 22: 1837- 1842.
- Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013; 12: 931-947.
- Liu Y, Lu Y, Celiku O, Li A, Wu Q, Zhou Y, et al. Targeting IDH1-mutated malignancies with NRF2 blockade. JNCI. 2019; 111: 1033-1041.
- Molenaar RJ, Maciejewski JP, Wilmink JW, van Noorden CJ. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene. 2018; 37: 1949-1960.
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360: 765- 773.
- Pansuriya TC, van Eijk R, d’Adamo P, van Ruler MA, Kuijjer ML, Oosting J, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet. 2011; 43: 1256-1261.
- Pirozzi CJ, Yan H. The implications of IDH mutations for cancer development and therapy. Nat Rev Clin Oncol. 2021; 18: 645-661.
- Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1α. Science. 2009; 324: 261-265.
- Xiang S, Gu H, Jin L, Thorne RF, Zhang XD, Wu M. LncRNA IDH1- AS1 links the functions of c-Myc and HIF1α via IDH1 to regulate the Warburg effect. Proc Natl Acad Sci. 2018; 115: E1465-E1474.
- Chen S, Wang Y, Xiong Y, Peng T, Lu M, Zhang L, et al. Wild-type IDH1 inhibits the tumor growth through degrading HIF-α in renal cell carcinoma. Int J Biol Sci. 2021; 17: 1250.
- Vaziri-Gohar A, Cassel J, Mohammed FS, Zarei M, Hue JJ, Hajihassani O, et al. Limited nutrient availability in the tumor microenvironment renders pancreatic tumors sensitive to allosteric IDH1 inhibitors. Nat Cancer. 2022; 3: 852-865.
- Ye J, Gu Y, Zhang F, Zhao Y, Yuan Y, Hao Z, et al. IDH1 deficiency attenuates gluconeogenesis in mouse liver by impairing amino acid utilization. Pro Natl Acad Sci. 2017; 114: 292-297.
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009; 324: 1029-1033.
- Harris AL. Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer. 2002; 2: 38-47.
- Carosi F, Broseghini E, Fabbri L, Corradi G, Gili R, Forte V, et al. Targeting Isocitrate Dehydrogenase (IDH) in Solid Tumors: Current Evidence and Future Perspectives. Cancers. 2024; 16: 2752.
- Gatto L, Franceschi E, Tosoni A, Di Nunno V, Maggio I, Lodi R, et al. IDH inhibitors and beyond: the cornerstone of targeted glioma treatment. Mol Diagnosis Therapy. 2021; 25: 457-473.
- Malta TM, Sabedot TS, Morosini NS, Datta I, Garofano L, Vallentgoed W, et al. The epigenetic evolution of glioma is determined by the IDH1 mutation status and treatment regimen. Cancer Res. 2024; 84: 741- 756.
- Liu WS, Chan SH, Chang HT, Li GC, Tu YT, Tseng HH, et al. Isocitrate dehydrogenase 1–snail axis dysfunction significantly correlates with breast cancer prognosis and regulates cell invasion ability. Breast Cancer Res. 2018; 20: 1-17.
- Xia W, Zhou J, Luo H, Liu Y, Peng C, Zheng W, et al. MicroRNA-32 promotes cell proliferation, migration and suppresses apoptosis in breast cancer cells by targeting FBXW7. Cancer Cell Int. 2017; 17: 1-11.
- Xia H, Long J, Zhang R, Yang X, Ma Z. MiR-32 contributed to cell proliferation of human breast cancer cells by suppressing of PHLPP2 expression. Biomed Pharmacother. 2015; 75: 105-110.
- Rostami B, Kahrizi S, Yekta BG, Ghadyani R, Keramatinia A, Hoseini SJ, et al. Correlation of IDH1 gene expression error in breast tumor biopsy in patients with invasive ductal carcinoma. Cell Mol Biol. 2024; 70: 242-247.
- Ghaedi H, Bastami M, Zare-Abdollahi D, Alipoor B, Movafagh A, Mirfakhraie R, et al. Bioinformatics prioritization of SNPs perturbing microRNA regulation of hematological malignancy-implicated genes. Genomics. 2015; 106: 360-366.
- Zarei M, Hue JJ, Hajihassani O, Graor HJ, Katayama ES, Loftus AW, et al. Clinical development of IDH1 inhibitors for cancer therapy. Cancer Treat Rev. 2022; 103: 102334.
- Minemura H, Takagi K, Sato A, Yamaguchi M, Hayashi C, Miki Y, et al. Isoforms of IDH in breast carcinoma: IDH2 as a potent prognostic factor associated with proliferation in estrogen-receptor positive cases. Breast Cancer. 2021; 28: 915-926.
- Piao S, Kim S, Seo Y, Lee J, Jeon S, Vu GH, et al. The relative isoform expression levels of isocitrate dehydrogenase in breast cancer: IDH2 is a potential target in MDA-MB-231 cells. Korean J Clin Oncol. 2023; 19: 60.
- Keyvani S, Karimi N, Orafa Z, Bouzari S, Oloomi M. Assessment of cytokeratin-19 gene expression in peripheral blood of breast cancer patients and breast cancer cell lines. Biomark Cancer. 2016; 8: 57-63
- Raynaud S, Carbuccia N, Colin C, Adélaïde J, Mozziconacci MJ, Metellus P, et al. Absence of R140Q mutation of isocitrate dehydrogenase 2 in gliomas and breast cancers. Oncol Lett. 2010; 1: 883- 884.
- Gaal J, Burnichon N, Korpershoek E, Roncelin I, Bertherat Jrm, Plouin PF, et al. Isocitrate dehydrogenase mutations are rare in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2010; 95: 1274-1278.
- Kang MR, Kim MS, Oh JE, Kim YR, Song SY, Seo SI, et al. Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer. 2009; 125: 353-355.
- Lopez GY, Reitman ZJ, Solomon D, Waldman T, Bigner DD, McLendon RE, et al. IDH1R132 mutation identified in one human melanoma metastasis, but not correlated with metastases to the brain. Biochem Biophy Res Commun. 2010; 398: 585-587.
- Gonthier K, Poluri RTK, Weidmann C, Tadros M, Audet-Walsh É. Reprogramming of isocitrate dehydrogenases expression and activity by the androgen receptor in prostate cancer. Mol Cancer Res. 2019; 17: 1699-1709.
- Gonthier K, Weidmann C, Berthiaume L, Jobin C, Lacouture A, Lafront C, et al. Isocitrate dehydrogenase 1 sustains a hybrid cytoplasmic– mitochondrial tricarboxylic acid cycle that can be targeted for therapeutic purposes in prostate cancer. Mol Oncol. 2023; 17: 2109- 2125.
- Han S, Liu Y, Cai SJ, Qian M, Ding J, Larion M, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020; 122: 1580-1589.
- Persico P, Lorenzi E, Losurdo A. Precision Oncology in Lower-Grade Gliomas: Promises and Pitfalls of Therapeutic Strategies Targeting IDH-Mutations. Cancers (Basel) 2022; 14: 1125.
- Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov. 2013; 3: 730-741.
- Reiter-Brennan C, Semmler L, Klein A. The effects of 2-hydroxyglutarate on the tumorigenesis of gliomas. Contemp Oncol. 2018; 22: 215-222.
- Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S, et al. Transformation by the (R)- enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012; 483: 484-488.
- Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012; 483: 479-483.
- Robertson KD. DNA methylation and human disease. Nat Rev Genet.2005; 6: 597-610.
- DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. NEJM. 2018; 378: 2386-2398.
- Mellinghoff IK, Penas-Prado M, Peters KB, Cloughesy TF, Burris HA, Maher EA, et al. Phase 1 study of AG-881, an inhibitor of mutant IDH1/IDH2, in patients with advanced IDH-mutant solid tumors, including glioma. J Clin Oncol. 2018; 36: 2002.
- Chen F, Bian K, Tang Q, Fedeles BI, Singh V, Humulock ZT, et al. Oncometabolites d-and l-2- hydroxyglutarate inhibit the AlkB family DNA repair enzymes under physiological conditions. Chem Res Toxicol. 2017; 30: 1102-1110.
- Wang P, Wu J, Ma S, Zhang L, Yao J, Hoadley KA, et al. Oncometabolite D-2-hydroxyglutarate inhibits ALKBH DNA repair enzymes and sensitizes IDH mutant cells to alkylating agents. Cell Rep. 2015; 13: 2353-2361.
- Sulkowski PL, Corso CD, Robinson ND, Scanlon SE, Purshouse KR, Bai H, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med. 2017; 9: eaal2463.
- Lu Y, Kwintkiewicz J, Liu Y, Tech K, Frady LN, Su YT, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. 2017; 77: 1709-1718.
- Sulkowski PL, Sundaram RK, Oeck S, Corso CD, Liu Y, Noorbakhsh S, et al. Krebs-cycle-deficient hereditary cancer syndromes are defined by defects in homologous-recombination DNA repair. Nat Genet. 2018; 50: 1086-1092.
- Ohba S, Mukherjee J, See WL, Pieper RO. Mutant IDH1-driven cellular transformation increases RAD51-mediated homologous recombination and temozolomide resistance. Cancer Res. 2014; 74: 4836-4844.
- Núñez FJ, Mendez FM, Kadiyala P, Alghamri MS, Savelieff MG, Garcia- Fabiani MB, et al. IDH1- R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci Transl Med. 2019; 11: eaaq1427.
- Waitkus MS, Diplas BH, Yan H. Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell. 2018; 34: 186-195.
- Tian W, Zhang W, Wang Y, Jin R, Wang Y, Guo H, et al. Recent advances of IDH1 mutant inhibitor in cancer therapy. Front Pharmacol. 2022; 13: 982424.
- Hansen E, Quivoron C, Straley K, Lemieux RM, Popovici-Muller J,Sadrzadeh H, et al. AG-120, an oral, selective, first-in-class, potent inhibitor of mutant IDH1, reduces intracellular 2HG and induces cellular differentiation in TF-1 R132H cells and primary human IDH1 mutant AML patient samples treated ex vivo. American Society of Hematology Washington, DC; 2014.
- Medeiros B, Fathi A, DiNardo C, Pollyea D, Chan S, Swords R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2017; 31: 272-281.
- Quivoron C, David M, Straley K, Travins J, Kim H, Chen Y, et al. AG-221, an oral, selective, first-in- class, potent IDH2-R140Q mutant inhibitor, induces differentiation in a xenotransplant model. Blood. 2014; 124: 3735.
- Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med. 2016; 21: 373-380.
- Kaminska B, Czapski B, Guzik R, Król SK, Gielniewski B. Consequences of IDH1/2 mutations in gliomas and an assessment of inhibitors targeting mutated IDH proteins. Molecules. 2019; 24: 968.
- Wang Y, Tang S, Lai H, Jin R, Long X, Li N, et al. Discovery of novel IDH1 inhibitor through comparative structure-based virtual screening. Front Pharmacol. 2020; 11: 579768.