Literature Review on Peyronie
- 1. Department of Urology B, Mohamed V University, Morocco
CITATION
Maachi Y, Boustani A, lalaoui AS, Slaoui A, Karmouni T, et al. (2024) Literature Review on Peyronie’s Disease. J Urol Res 11(3): 1156.
INTRODUCTION
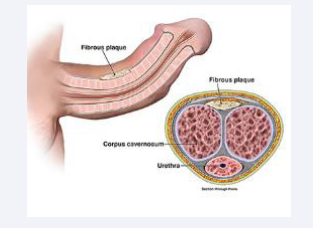
Peyronie’s Disease (PD) is a complex condition affecting the penis, characterized by the development of fibrous plaques within the tunica albuginea [Figure 1].
Figure 1: Development of fibrous plaques within the tunica albuginea
This leads to penile curvature, pain, and often erectile dysfunction. This review covers etiology, clinical presentation, diagnosis, treatment options, psychological impact, and emerging therapies.
Prevalence ans incidence
- Prevalence
The prevalence of Peyronie’s disease varies according to different studies but is estimated to be between 0.4% and 20.3% among adult men. The wide range is often due to differences in diagnostic methods and populations studied.
- Age
The condition is most common in men aged 50 to 60 years. However, it can occur at any age, although its prevalence increases with age.
- Incidence
The exact incidence is difficult to determine due to underdiagnosis and patients’ reluctance to seek medical advice for genital symptoms. However, studies have reported an annual incidence of 0.3% to 0.7%.
These data indicate that Peyronie’s disease is relatively common, especially among older men, and is often associated with other medical conditions. Patients with risk factors or comorbidities should be closely monitored for early detection and treatment.
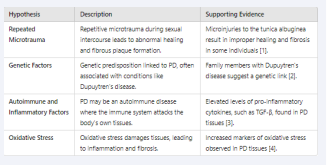
Etiology and Pathogenesis
The exact cause of Peyronie’s Disease remains uncertain, but
several hypotheses have been proposed:
- Repeated Microtrauma
Repetitive microtrauma during sexual intercourse can lead to abnormal scarring and fibrous plaque formation. The tunica albuginea, an elastic structure surrounding the penile corpora cavernosa, undergoes micro-injuries that, in some individuals, do not heal properly, leading to fibrosis [1].
- Genetic Factors
Studies show a genetic predisposition in PD patients. For example, the presence of Dupuytren’s disease (palmar fibromatosis) in family members suggests a common genetic basis [2].
- Autoimmune and Inflammatory Factors
Some researchers speculate that PD could be an autoimmune disease where the immune system attacks the body’s own tissues. Elevated levels of pro-inflammatory cytokines, such as TGF-β, have been found in PD tissues [3].
- Oxidative Stress
Oxidative stress also plays a key role. Free radicals can damage tissues, leading to inflammation and fibrosis. Studies have shown increased markers of oxidative stress in PD tissues [4] [Table 1].
Table 1: Etiology and Pathogenesis of Peyronie’s Disease
CLINICAL PRESENTATION
PD progresses through two main phases:
Acute (Inflammatory) Phase
- Duration
Typically lasts from 6 to 18 months.
- Symptoms
Pain during erections and even at rest, progressive penile curvature, formation of palpable nodules or plaques.
- Progression
This is the phase where penile deformity develops most rapidly [5].
Chronic Phase
- Duration
Indefinite, often lifelong.
- Symptoms
Stabilization of curvature, disappearance of pain, persistence of plaques, and often erectile dysfunction.
- Impact
The chronic phase is characterized by stabilization of deformation, although the psychological and functional impact can be significant [6].
- Diagnostic
Diagnosis of PD primarily relies on clinical evaluation:
MEDICAL HISTORY
Recollecting a detailed history of symptoms, including duration, progression, and impact on sexual relationships.
Examining the history of penile trauma or Dupuytren’s disease.
Physical Examination
Palpation of the penis to identify plaques or nodules.
Evaluating penile curvature during induced erection with photographs or self-injection of erectogenic drugs.
Imaging
Doppler Ultrasound: Used to visualize plaques and assess penile blood flow. It helps determine plaque size, location, and extension [7].
MRI: Sometimes used in complex cases to provide additional details on plaque structure and involvement of surrounding tissues [8].
TREATMENT OPTIONS
Non-Surgical Treatments
Oral Medications
- Potassium Para-aminobenzoate (Potaba)
Anti-fibrotic, although its efficacy is debated and often limited by gastrointestinal side effects [9].
- Pentoxifylline
Improves microcirculation and has anti-fibrotic effects. Preliminary studies show a reduction in curvature and plaques [10].
- Phosphodiesterase Type 5 Inhibitors (PDE5i)
Tadalafil and other PDE5i may help improve erectile function and reduce fibrosis progression by increasing tissue perfusion [11].
Intralesional Injections
Collagenase Clostridium histolyticum (CCH): FDA-approved for treating moderate to severe PD. Clinical trials show significant improvement in curvature and symptoms [12].
- Interferon-alpha 2b
Reduces collagen synthesis and fibroblast proliferation.
Clinical trials show moderate reduction in curvature [13].
- Verapamil
Calcium channel blocker, reduces collagen synthesis. Studies show some efficacy in plaque reduction [14].
- Topical Therapies and Iontophoresis
Verapamil and other locally applied agents have limited results. Iontophoresis enhances penetration of these drugs, but evidence of their effectiveness remains weak [15].
Shockwave Therapy
- Low-Intensity Shockwaves
Potential to reduce pain and plaque size. However, study results are mixed, and clinical use remains experimental [16].
Surgical Treatments
Surgical interventions are reserved for patients with significant deformity or failure of conservative treatments.
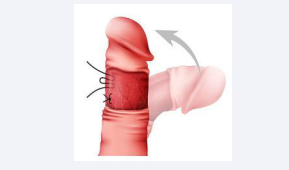
Plication Procedures [Figure 2]
Figure 2: Plication Procedures
- Nesbit Technique
Resection of small segments of the tunica albuginea on the opposite side of curvature to shorten the penis and straighten the curvature. Effective but can lead to penile shortening [17].
- Yachia Technique
Modification of the Nesbit method, with longitudinal incisions sutured transversely. Less risk of post-operative erectile dysfunction [18].
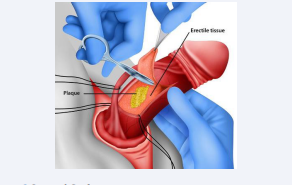
Plaque Incision/Excision and Grafts [Figure 3]
Figure 3: Surgical Graft
- Plaque Incision or Excision
Performed for severe curvatures. The plaque is incised or excised, followed by grafting (e.g., saphenous vein, bovine pericardium) to restore penile length and straightness [19].
- Grafts
Use of autologous, allogeneic, or synthetic materials to fill the defect after plaque excision [20].
Penile Prosthesis Implantation
Inflatable or Semi-rigid Prostheses: Indicated in patients with severe erectile dysfunction. Implants correct curvature and provide the rigidity needed for sexual intercourse. Patients report high post-surgical satisfaction [21].
PSYCHOLOGICAL IMPACT
PD has a significant psychological impact :
- Depression and Anxiety
Deformity and pain can lead to feelings of shame and depression. Fear of sexual failure and relationship problems are common [22].
- Relational Issues
PD affects not only the individual but also the partner. Open communication and couple counseling can be beneficial [23].
- Counseling and Support Groups
Important for helping patients manage the emotional impact of the disease. Cognitive-behavioral therapies (CBT) can be effective [24].
EMERGING THERAPIES
Recent research explores new therapeutic avenues
- Gene Therapy
Potential to correct underlying genetic abnormalities. Studies are still in preliminary stages [25].
- Stem Cell Therapy
Mesenchymal stem cells have shown anti-fibrotic effects in preclinical models. Human clinical trials are underway [26].
Innovative Pharmacological Agents
Development of new drugs targeting specific molecular pathways involved in fibrosis. For example, TGF-β inhibitors show promise [27].
CONCLUSION
Peyronie’s Disease is a complex condition requiring a multifaceted approach to treatment. A thorough understanding of its etiology, precise clinical evaluation, and tailored therapeutic options are essential to improve outcomes for patients.
REFERENCES
- Somers KD, Dawson DM. Fibrin deposition in Peyronie’s disease plaque. J Urol. 1997; 157: 311-315.
- Devine Jr CJ , Somers KD, Jordan SG, Schlossberg SM. Proposal: trauma as the cause of the Peyronie’s lesion. J Urol. 1997; 157: 285-290.
- Gonzalez-Cadavid NF, Rajfer J. Mechanisms of Disease: new insights into the cellular and molecular pathology of Peyronie’s disease. Nat Clin Pract Urol. 2005; 2: 291-297.
- El-Sakka AI, Hassoba HM, Chui RM, Bhatnagar RS, Lue TF. An animal model of Peyronie’s-like condition associated with an increase of transforming growth factor beta mRNA and protein expression. J Urol. 1997; 158: 2284-2290.
- Levine LA, Greenfield JM. Establishing a standardized evaluation of the man with Peyronie’s disease.Int J Impot Res. 2003; 15: 103-112.
- Gelbard MK, DoreyF, James K. The natural history of Peyronie’s disease. J Urol. 1990; 144: 1376-1379.
- Prieto Castro R., Leibar Tamayo A., Casado Pérez C, Fernández Pérez C, Pichardo Franco C. “Clinical and ultrasound study of the fibrous plaque in the initial phase of Peyronie’s disease.” *Archivos Españoles de Urología*. 2003 ; 56 : 369-376.
- Tsambarlis P, Levine LA. “Nonsurgical Management of Peyronie’s Disease: Review and State of the Art.” *Translational Andrology and Urology. 2018 ; 7 : 603-615.
- Pryor JP, Farrell CF. “Controlled clinical trial of vitamin E in Peyronie’s disease.” *Brit J Urol. 1983 ; 55 : 204-206.
- Smith JF, Brant WO, Fradet V. Pentoxifylline treatment and the progression of Peyronie’s disease.” Int J Impotence Res. 2011 ; 23 : 202-206.
- Abern MR, Larsen S, Levine LA. Combination of penile traction, intralesional verapamil, and oral therapies for Peyronie’s disease. J Sex Med. 2012; 9: 288-295.
- Gelbard M, Goldstein I, Hellstrom WJG, McMahon CG, Smith T, Tursi J, et al. Clinical efficacy, safety, and tolerability of collagenase clostridium histolyticum for the treatment of Peyronie’s disease in two large double-blind, randomized, placebo-controlled phase 3 studies. J Sex Med. 2013; 10: 199-207.
- Hellstrom WJG, Kendirci M, Matern R, Cockerham Y, Myers L, Sikka SC, et al. Single-blind, multicenter, placebo controlled, parallel study to assess the safety and efficacy of intralesional interferon alpha-2B for minimally invasive treatment for Peyronie’s disease. J Urology. 2006; 176: 394-398.
- Levine LA, Estrada CR. Intralesional verapamil for the treatment of Peyronie’s disease: a review. Int J Impot Res. 2002; 24: 324-328.
- Shirazi M, Haghpanah AR, Badiee M, Afrasiabi MA, Haghpanah S. Efficacy of intralesional verapamil for treatment of Peyronie’s disease: a randomized single-blind, placebo-controlled study. Int Urol Nephrol. 2009; 41: 359-363.
- Palmieri A, Imbimbo C, Longo N. Penile extenders for the treatment of Peyronie’s disease: a preliminary study.” BJU Int. 2009 ; 103 : 912- 917.
- Nesbit RM. Congenital curvature of the phallus: report of three cases with description of corrective operation. J Urol. 1965; 93: 230-232.
- Yachia D. Modified corporoplasty for the treatment of penile curvature. J Urol. 1990; 143: 80-82.
- Gelbard M, Hayden B, Phillips J. Venous patch grafting for Peyronie’s disease. Long-term results.” J Urol. 1995 ; 154 : 1457-1459.
- Kadioglu A, Sanli O, Akman T, Gurkan L, Cakan M, Celtik M. Surgical treatment of Peyronie’s disease: a critical analysis. Eur Urol. 2006; 53: 720-728.
- Montorsi F, Rigatti P, Carmignani G. Corporal rotation and venous patch grafting for Peyronie’s disease with severe penile shortening.” European Urol. 2000 ; 38 : 391-395.
- Nelson CJ, Diblasio C, Kendirci M, Hellstrom W, Guhring P, Mulhall JP. The chronology of depression and distress in men with Peyronie’s disease. J Sex Med. 2008; 5: 1985-1990.
- Gelbard MK, Dorey F, James K. The natural history of Peyronie’s disease. J Urol. 1990; 144:1376-1379.
- Hallam-Jones R, Wylie KR, Eardley I. Psychological and relationship problems in men with Peyronie’s disease.” J Sexual Med. 2001 ; 16 : 152-155.
- Rehman J, Benet AE, Melman A. Use of gene transfer in the treatment of Peyronie’s disease.” Int J Impotence Res. 1997 ; 9 : 181-184.
- Gokce A, Abd Elmageed ZY, Lasky J. Effects of patient characteristics and disease variables on clinical outcome in Peyronie’s disease treated with intralesional interferon-alpha-2b.” J Sexual Med. 2016 ; 13 : 795-803.
- Sikka SC, Hellstrom WJG. Role of oxidative stress and antioxidants in Peyronie’s disease. Int J Impot Res. 2002; 14: 353-360.